TZHASY210
Steritest® NEO Device
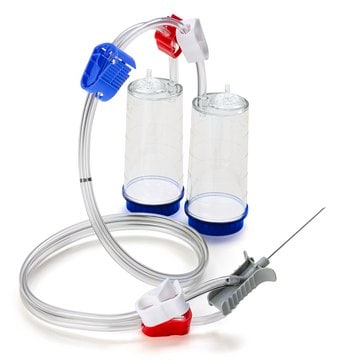
For liquids in syringes. Blue base canister with vented double needle adapter for sequential testing of syringe contents and needle surfaces.
Synonym(s):
Blue Base Steritest® NEO device for sterility testing, Sterility testing device, membrane filtration device, membrane filtration canister, closed membrane filtration
About This Item
Recommended Products
material
Nylon 66 adapter (for needle)
PVC tubing (double lumen)
mixed cellulose esters (MCE) membrane
stainless steel (for needle)
styrene-acrylonitrile (SAN) (For Canister)
Quality Level
agency
EP (2.6.1)
JP (4.06)
USP 71
sterility
sterile; γ-irradiated
manufacturer/tradename
Steritest®
packaging
pkg of 10 blisters per box, Single packed
parameter
120 mL sample volume (graduation marks at 25, 50, 75 and 100 mL)
3.1 bar max. inlet pressure (45 psi) at 25 °C
45 °C max. temp.
tubing L
850 mm
color
blue Canister Base
matrix
MF-Millipore™
pore size
0.45 μm pore size
input
sample type pharmaceutical(s)
liquid
application(s)
pharmaceutical
sterility testing
shipped in
ambient
General description
Steritest® NEO Device is a membrane filtration device for sterility testing of filterable pharmaceutical products. The device simplifies every aspect of testing, from handling to traceability. This device ensures that pharmaceutical products are never exposed to the environment during the testing process. The closed system minimizes false positives and offers the highest levels of quality and reliability. This test system offers an optimized and fully regulatory compliant testing process, when used with the Steritest® Symbio pump, specific accessories and high-quality culture media and rinsing fluids. The device comes with vented double needle. The adapter allows for sequential testing of syringe contents and needle surfaces. The system has two blue based canisters with mixed cellulose esters (MCE) membrane, providing an optimal filtration flow rate for standard products.
Application
Features and Benefits
- One-stop-shop for sterility testing with our devices, pumps, media, fluids, and services
- Steritest® devices are manufactured in our Center of Excellence in Molsheim, France, with high-quality control standards maintaining the Certificate of Quality for each lot.
- New needle design
The design of this new needle has been optimized with short needle length, grips, and ridges to offer dexterity and security to the operator while piercing the small container - Smarter workflow
The new Steritest® NEO cartridge device benefits fromall the improvements such as colored clamps, graduations for accurate volume measurement, optimized identification, and traceability with the new peel-off label - Completely closed set up
Pharmaceutical products are never exposed to the environment during the testing process with Steritest® NEO devices. Filtration, rinsing, media addition and incubation are conducted within a closed system. - Consistent performance
100% integrity testing, strict physical and microbiological tests performed at every step of the assembly of the Steritest® NEO device before release from manufacturing. - New tubing disconnection tool
Packaging
Legal Information
configured for
related product
required but not provided
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
The new Velax® cutting clamp to sever the tubing of a Steritest® NEO device makes sterility testing by membrane filtration safer and more convenient than ever before.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service