TZHVCA210
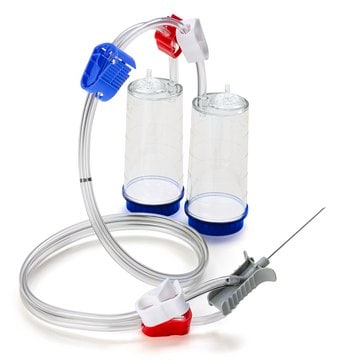
Steritest® NEO Device
For liquids in cartridges and small soft plastic containers. Red base canister comes with a single short (20 mm) needle.
Synonym(s):
Red Base Steritest® NEO device for sterility testing, Sterility testing device, membrane filtration device, membrane filtration canister, closed membrane filtration
About This Item
Recommended Products
material
Nylon 66 adapter (for needle)
PVC tubing (double lumen)
PVDF membrane
stainless steel (for needle)
styrene-acrylonitrile (SAN) (for canister)
agency
EP 2.6.1
JP 4.06
USP 71
sterility
sterile; γ-irradiated
manufacturer/tradename
Steritest®
packaging
pkg of 10 blisters per box (Single packed)
parameter
120 mL sample volume (graduation marks at 25, 50, 75 and 100 mL)
3.1 bar max. inlet pressure (45 psi) at 25 °C
45 °C max. temp.
tubing L
850 mm
color
red Canister Base
matrix
Durapore®
pore size
0.45 μm pore size
input
liquid
pharmaceutical(s)
application(s)
pharmaceutical
sterility testing
compatibility
for use with Steritest® Symbio FLEX Pump Kit, 2 media (SYMBFLE01)
for use with Steritest® Symbio ISL Pump Kit, 2 media (SYMBISL01)
for use with Steritest® Symbio LFH Pump Kit (SYMBLFH01)
General description
Steritest® NEO is a membrane filtration device for sterility testing of filterable pharmaceutical products. The device simplifies every aspect of testing, from handling to traceability. The closed system minimizes false positives and offers the highest levels of quality and reliability. This device ensures that pharmaceutical products are never exposed to the environment during the testing process. This test system offers an optimized and fully regulatory compliant testing process when used with the Steritest® Symbio pump, specific accessories and high-quality culture media and rinsing fluids. The Steritest® NEO device for liquids in cartridge includes a short (20 mm) single needle for easy and safe access to cartridge or small soft plastic container and a separate vent needle. The red canister base indicates low adsorption Durapore® Poly vinylidene fluoride (PVDF) membrane and specific drain design. This optimizes the rinsing of products that inhibit the microbial growth.
Application
Features and Benefits
- One-stop-shop for sterility testing with our devices, pumps, media, fluids, and services
- Steritest® devices are manufactured in our Center of Excellence in Molsheim, France, with high-quality control standards maintaining the Certificate of Quality for each lot.
- New needle design
- Smarter workflow
- Completely closed set up
- Consistent performance
- New tubing disconnection tool
Packaging
Legal Information
configured for
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Related Content
The new Velax® cutting clamp to sever the tubing of a Steritest® NEO device makes sterility testing by membrane filtration safer and more convenient than ever before.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service