00410
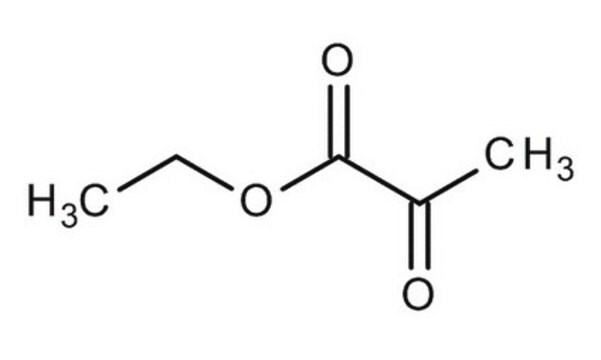
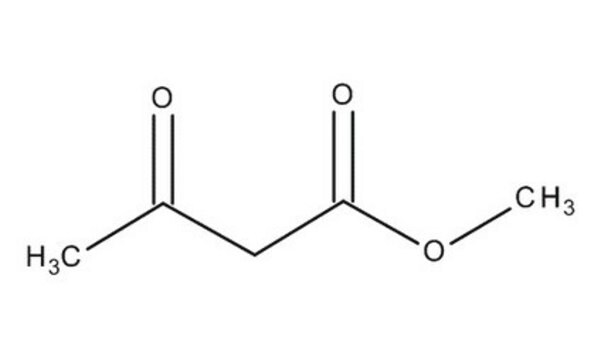
Ethyl acetoacetate
puriss. p.a., ≥99.0% (GC)
Synonym(s):
Acetoacetic ester
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(1)
Select a Size
Change View
About This Item
Linear Formula:
CH3COCH2COOC2H5
CAS Number:
Molecular Weight:
130.14
Beilstein/REAXYS Number:
385838
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39022303
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
4.48 (vs air)
Quality Level
vapor pressure
1 mmHg ( 28.5 °C)
grade
puriss. p.a.
assay
≥99.0% (GC)
autoignition temp.
580 °F
expl. lim.
9.5 %
impurities
≤0.5% water
refractive index
n20/D 1.419
bp
181 °C (lit.)
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Ethyl acetoacetate can be used as a reactant in the synthesis of:
It can be also utilized as a reactant in the transesterification and asymmetric hydrogenation reactions to produce valuable products.
- Knoevenagel condensation products by reacting with α-alkylideneacetoacetate and aliphatic, aromatic, heteroaromatic aldehydes.
- Michael adducts, via Michael addition reaction with chalcones and azachalcones in the presence of a base catalyst.
- Substituted nicotinic acid derivatives by treating with α, β-unsaturated oximes via Michael addition followed by ring closure reaction.
It can be also utilized as a reactant in the transesterification and asymmetric hydrogenation reactions to produce valuable products.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
flash_point_f
164.3 °F - closed cup
flash_point_c
73.5 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Knoevenagel condensation reaction between benzaldehyde and ethyl acetoacetate in microreactor and membrane microreactor
Lau WN, et al.
Microporous and Mesoporous Materials : The Official Journal of the International Zeolite Association, 115(1-2), 156-163 (2008)
Michael addition of ethyl acetoacetate to α,β -unsaturated oximes in the presence of FeCl3: a novel synthetic route to substituted nicotinic acid derivatives
Chibiryaev AM, et al.
Tetrahedron Letters, 41(41), 8011-8013 (2000)
Mechanochemical Michael reactions of chalcones and azachalcones with ethyl acetoacetate catalyzed by K2CO3 under solvent-free conditions
Zhang Ze, et al.
Chemistry Letters (Jpn), 33(2), 168-169 (2004)
Boric acid: an efficient and environmentally benign catalyst for transesterification of ethyl acetoacetate
Kondaiah GCM, et al.
Tetrahedron Letters, 49(1), 106-109 (2008)
Enantioselective catalytic asymmetric hydrogenation of ethyl acetoacetate in room temperature ionic liquids
Berthod M, et al.
Tetrahedron Asymmetry, 15(14), 2219-2221 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service