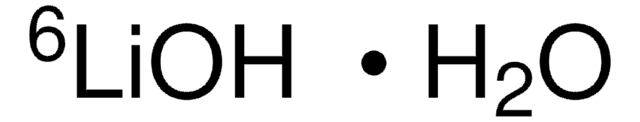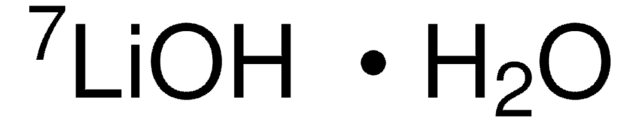402974
Lithium hydroxide monohydrate
ACS reagent, ≥98.0%
About This Item
Recommended Products
grade
ACS reagent
assay
≥98.0%
form
powder, crystals or granules
impurities
≤0.01% insolubles
≤2.0% Li2CO3
pH
12 (0.4 g/L)
solubility
water: soluble 216 g/L at 20 °C
anion traces
chloride (Cl-): ≤0.01%
sulfate (SO42-): ≤0.05%
cation traces
Fe: ≤0.002%
heavy metals: ≤20 ppm (by ICP-OES)
storage temp.
room temp
SMILES string
[Li+].O.[OH-]
InChI
1S/Li.2H2O/h;2*1H2/q+1;;/p-1
InChI key
GLXDVVHUTZTUQK-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
Application
It may be used in the synthesis of the following:
- Lithium titanate (Li4Ti5O12) heterogeneous nanostructures.
- Tin oxide-carbon (SnO2/C) nanocomposites.
- Zinc oxide (ZnO) nanowire arrays.
- Lithium diborate gel films.
- Porous layered lithium cobalt oxide (LiCoO2) films.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
Storage Class
8B - Non-combustible corrosive hazardous materials
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Experts discuss challenges and production processes of nickel-rich layered oxide cathode materials in energy storage systems.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service