About This Item
Recommended Products
grade
ACS reagent
Quality Level
agency
suitable for SM 4500 - NH3
suitable for SM 5210
vapor pressure
2.6 mmHg ( 20 °C)
assay
≥99.5%
form
powder
technique(s)
PCR: suitable
impurities
≤0.005% Insoluble in methanol
≤0.05% Nonvolatile with methanol
mp
160 °C (dec.) (lit.)
solubility
water: soluble
Looking for similar products? Visit Product Comparison Guide
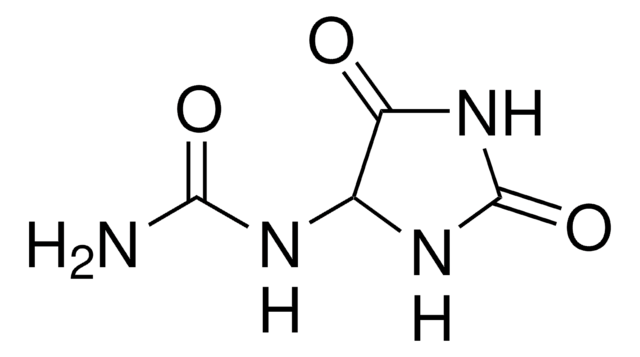
General description
Application
- N-substituted cinnamamides via amidation reaction between cinnamic acid and benzylamines.
- β-Aminoalcohols through the regioselective epoxides ring opening with aromatic amines in the presence of glycerol as a co-catalyst.
- Organosulfur compounds via thia-Michael addition of thiols to α, β-unsaturated compounds.
signalword
Danger
hcodes
Hazard Classifications
Repr. 1B
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Information on Isoelectric Focusing including what it is and how it is used. In order to ensure the high performance of analysis, isoelectric point (pI) standards are needed.
Acid and base chart lists the strength of acids and bases (strongest to weakest) in order. Simple to use laboratory reference chart for scientists, researchers and lab technicians.
Related Content
This page is intended to make it easier to find the consumables you need based on the analytical method you’re using. Methods included on this page come from the EPA, Standard Methods and ASTM.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service