B85919
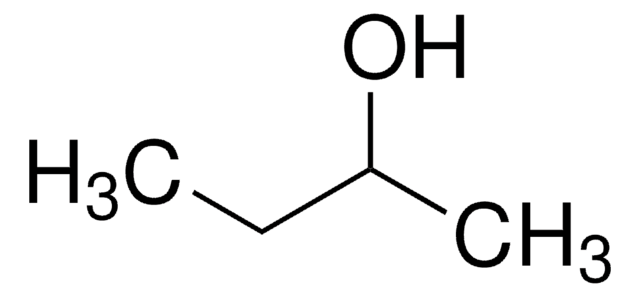
2-Butanol
ReagentPlus®, ≥99%
Synonym(s):
sec-Butyl alcohol
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(2)
Select a Size
Change View
About This Item
Linear Formula:
CH3CH2CH(OH)CH3
CAS Number:
Molecular Weight:
74.12
Beilstein/REAXYS Number:
773649
EC Number:
MDL number:
UNSPSC Code:
12352001
PubChem Substance ID:
NACRES:
NA.21
assay:
≥99%
bp:
98 °C (lit.)
vapor pressure:
12.5 mmHg ( 20 °C)
Recommended Products
vapor density
2.6 (vs air)
Quality Level
vapor pressure
12.5 mmHg ( 20 °C)
product line
ReagentPlus®
assay
≥99%
form
liquid
autoignition temp.
761 °F
expl. lim.
9.8 %
IVD
for in vitro diagnostic use
dilution
(for general lab use)
Looking for similar products? Visit Product Comparison Guide
General description
2-Butanol is secondary alcohol mainly used as a solvent in organic synthesis. 2-butanol readily converts into 2-butanone (methyl ethyl ketone, MEK), which is used as a solvent in the industrial sector and many domestic cleaning products. It is an intermediate in devulcanizing rubber and the production of alkyl ester for use as biodiesel fuel.
Application
2-Butanol is used:
- As a precursor to produce 2-butanone in presence of KMnO4 oxidant and CPC (N-cetylpyridinium chloride) micellar catalyst.
- In the production of CH3NH3PbI3 perovskite films.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - STOT SE 3
target_organs
Central nervous system, Respiratory system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
flash_point_f
80.6 °F - closed cup
flash_point_c
27 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mark B Shiflett et al.
The journal of physical chemistry. B, 110(29), 14436-14443 (2006-07-21)
In this article, we investigate vapor-liquid-liquid equilibria (VLLE) of binary systems using a simple volumetric method. Being different from the usual cloud-point method for the determination of liquid-liquid separation boundaries, the present volumetric method is able to determine the direct
Tantalum compounds as heterogeneous catalysts for saccharide dehydration to 5-hydroxymethylfurfural.
Fengli Yang et al.
Chemical communications (Cambridge, England), 47(15), 4469-4471 (2011-03-04)
A new solid acid, based on tantalum hydroxide, was used to catalyze saccharide dehydration into 5-hydroxymethylfurfural (HMF) with high catalytic activity and excellent stability in a water-2-butanol biphasic system. Furthermore, good results were also obtained from Jerusalem artichoke juice with
Dagmara Wojtków et al.
The journal of physical chemistry. A, 110(36), 10552-10557 (2006-09-08)
The effect of temperature and concentration on the structure of sec-butyl alcohol and isobutyl alcohol/water binary mixtures in the alcohol-rich region (mole fraction of water X(H2O) < 0.3) has been studied using Fourier transform (FT) near-infrared (NIR) spectroscopy. The experimental
Ravi K Pathak et al.
Environmental science & technology, 46(21), 11660-11669 (2012-09-19)
Limonene has a strong tendency to form secondary organic aerosol (SOA) in the atmosphere and in indoor environments. Initial oxidation occurs mainly via ozone or OH radical chemistry. We studied the effect of O(3) concentrations with or without a OH
Robert Brosnan et al.
Anesthesia and analgesia, 103(1), 86-91 (2006-06-23)
Chirality has been proposed as a means for distinguishing relevant from irrelevant molecular targets of action, but the sensitivity and specificity of this test is unknown for volatile anesthetics. We applied enantiomers of two chiral anesthetic alcohols (2-butanol and 2-pentanol)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service