N4882
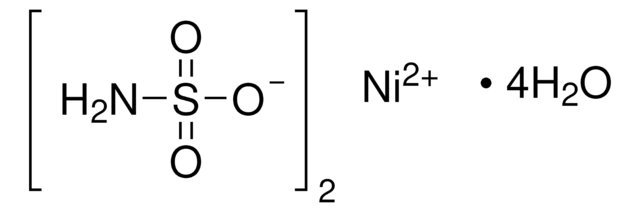
Nickel(II) sulfate hexahydrate
ReagentPlus®, powder or crystals
About This Item
Recommended Products
vapor density
2.07 (vs air)
Quality Level
product line
ReagentPlus®
assay
98.0-102.0% (EDTA titration)
form
powder or crystals
cation traces
Fe: ≤0.001%
SMILES string
O.O.O.O.O.O.[Ni++].[O-]S([O-])(=O)=O
InChI
1S/Ni.H2O4S.6H2O/c;1-5(2,3)4;;;;;;/h;(H2,1,2,3,4);6*1H2/q+2;;;;;;;/p-2
InChI key
RRIWRJBSCGCBID-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
General description
Application
- As a catalyst for the synthesis of α-aminophosphonates by a condensation reaction of aromatic aldehydes, primary amines, and diethylphosphite.
- In the synthesis of nickel (II) hexamethylenetetramine complex [Ni(HMTA)2 (NCS)2(H2O)2]H2O with thiocyanate coligand.
- As a nickel source in the synthesis of nanosized nickel phosphides by the solvothermal route using red phosphorus as the P-3 precursor.
Legal Information
signalword
Danger
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1A Inhalation - Muta. 2 - Repr. 1B - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT RE 1 Inhalation
target_organs
Respiratory Tract
Storage Class
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service