70215
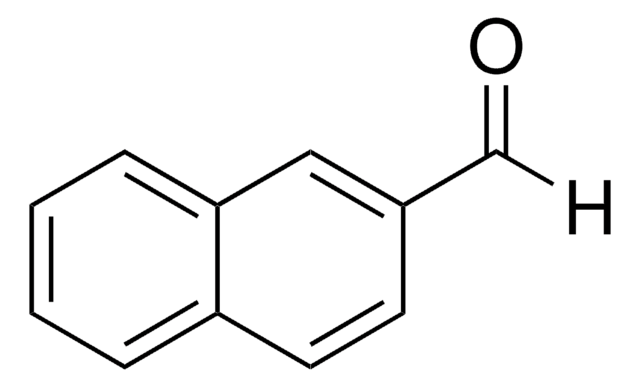
2,3-Naphthalenedicarboxaldehyde
suitable for fluorescence
Synonym(s):
NDA
Select a Size
Select a Size
About This Item
Recommended Products
form
crystals
Quality Level
impurities
≤2% mono- and dicarboxylic acid (1H-NMR)
mp
131-133 °C (lit.)
132-135 °C
fluorescence
λex 420 nm; λem ~480 nm in 0.1 M borate pH 9.3 (after derivatization with glycine [~90 μM glycine, ~20 μM N-])
suitability
suitable for fluorescence
storage temp.
2-8°C
SMILES string
[H]C(=O)c1cc2ccccc2cc1C([H])=O
InChI
1S/C12H8O2/c13-7-11-5-9-3-1-2-4-10(9)6-12(11)8-14/h1-8H
InChI key
ZIPLKLQPLOWLTM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
1 of 4
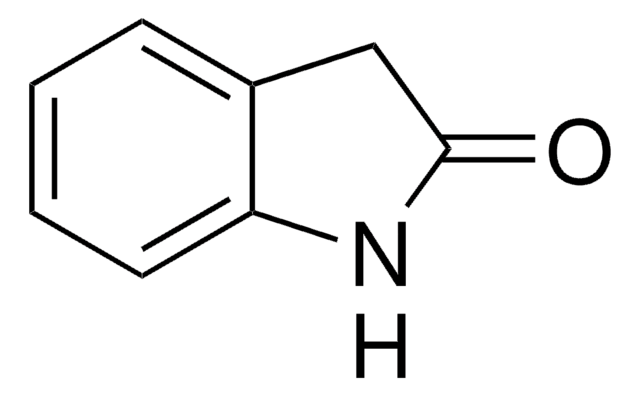
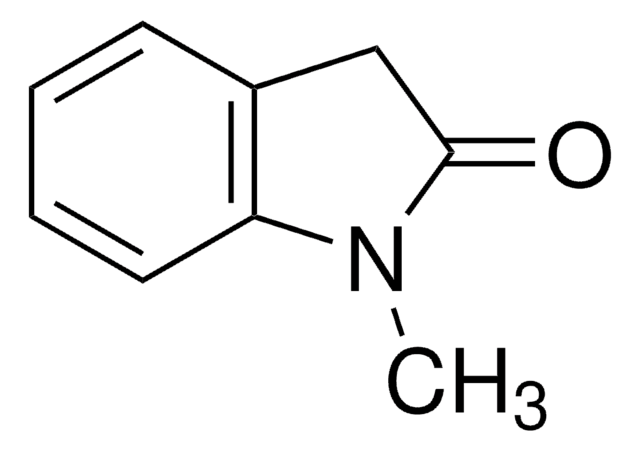
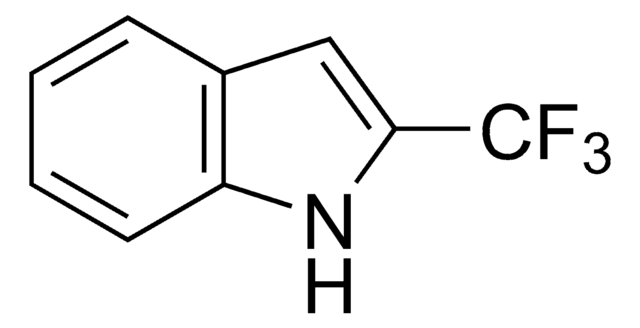
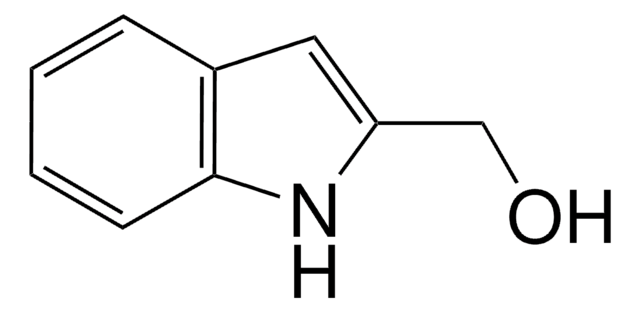
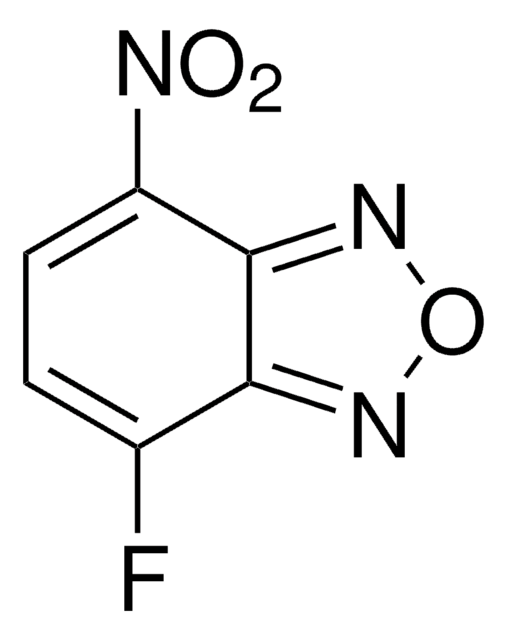
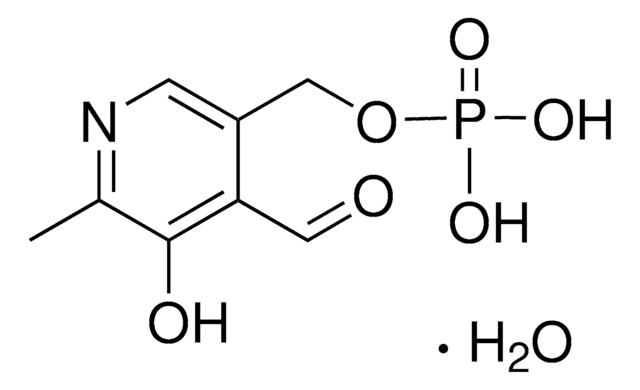
This Item | 466921 | 759937 | 687227 |
|---|---|---|---|
| Quality Level 100 | Quality Level 100 | Quality Level 100 | Quality Level 100 |
| form crystals | form solid | form solid | form solid |
| mp 123-128 °C (lit.) | mp 85-88 °C (lit.) | mp 106-110 °C | mp 72-78 °C |
| bp 227 °C/73 mmHg (lit.) | bp - | bp - | bp - |
| Gene Information human ... PGR(5241) | Gene Information - | Gene Information - | Gene Information - |
General description
2,3-Naphthalenedicarboxaldehyde is a fluorescent derivatization agent of primary amines, amino acids, and small peptides. The reaction between the amino compounds and NDA results in highly fluorescent and stable derivative compounds.
Application
Features and Benefits
- High Reaction rate.
- High Fluorescence quantum yield.
- No uncommon excitation wavelength.
- No side reactions.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)

![1,5,7-Triazabicyclo[4.4.0]dec-5-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/171/446/333d560c-cff6-4958-b489-5acfb3057cce/640/333d560c-cff6-4958-b489-5acfb3057cce.png)