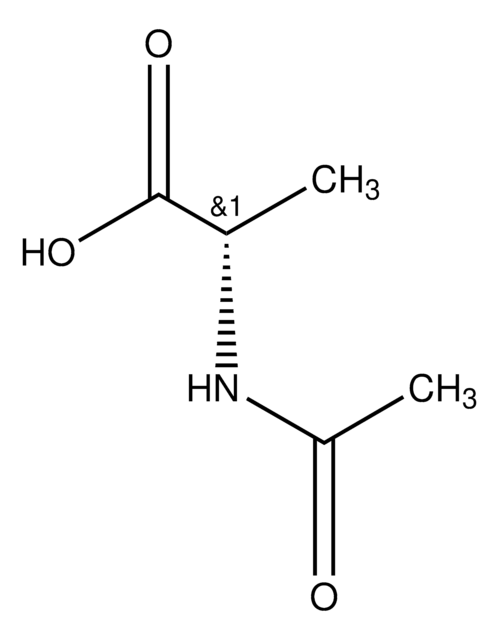
A1501
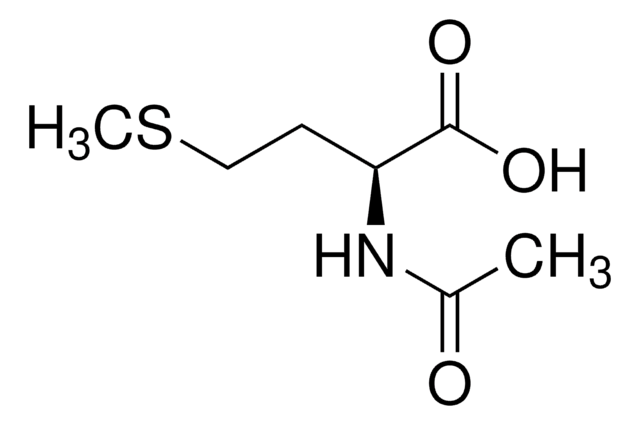
N-Acetyl-D-methionine
~99%, suitable for ligand binding assays
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(1)
Select a Size
Change View
About This Item
Empirical Formula (Hill Notation):
C7H13NO3S
CAS Number:
Molecular Weight:
191.25
Beilstein/REAXYS Number:
1725553
EC Number:
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
N-Acetyl-D-methionine, ~99%
assay
~99%
Quality Level
form
powder or crystals
technique(s)
ligand binding assay: suitable
color
white
mp
102.3-103.6 °C
storage temp.
−20°C
SMILES string
CSCC[C@@H](NC(C)=O)C(O)=O
InChI
1S/C7H13NO3S/c1-5(9)8-6(7(10)11)3-4-12-2/h6H,3-4H2,1-2H3,(H,8,9)(H,10,11)/t6-/m1/s1
Looking for similar products? Visit Product Comparison Guide
Application
N-Acetyl-D-methionine may be used as a substrate to identify, differentiate and characterized N-acylamino acid racemase(s) and N-acyl-D-amino acid amidohydrolase(s).
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Pei-Hsun Lin et al.
European journal of biochemistry, 269(19), 4868-4878 (2002-10-02)
An N-acyl-d-amino acid amidohydrolase (N-D-AAase) was identified in cell extracts of a strain, Iso1, isolated from an environment containing N-acetyl-d-methionine. The bacterium was classified as Variovorax paradoxus by phylogenetic analysis. The gene was cloned and sequenced. The gene consisted of
Wen-Ching Wang et al.
Journal of molecular biology, 342(1), 155-169 (2004-08-18)
N-acylamino acid racemase (NAAAR) catalyzes the racemization of N-acylamino acids and can be used in concert with an aminoacylase to produce enantiopure alpha-amino acids, a process that has potential industrial applications. Here we have cloned and characterized an NAAAR homologue
K Lertratanangkoon et al.
Toxicology and applied pharmacology, 122(2), 191-199 (1993-10-01)
Bromobenzene (800 mg/kg, ip) caused severe liver necrosis with massive hemorrhage in the golden Syrian hamster within the first 24 hr. Kidney injury was also observed. Treatment with N-acetylmethionine (NAM) at an ip dose of 1200 mg/kg at 5 hr
S Pittelkow et al.
Protein expression and purification, 12(2), 269-276 (1998-03-31)
Aminoacylase I (EC 3.5.1.14) is one of the most abundant enzymes in the cortical region of mammalian kidney. Both the porcine and the human enzyme were overexpressed using baculovirus expression vector systems and purified by hydrophobic interaction chromatography and anion-exchange
M Sugumaran et al.
Archives of insect biochemistry and physiology, 38(1), 44-52 (1998-05-20)
Incubation of catechol with mushroom tyrosinase in the presence of N-acetylmethionine resulted in the generation of an adduct. This product was identified to be N-acetylmethionyl catechol, on the basis of spectral characteristics and well-characterized chemical reaction of o-benzoquinone with N-acetylmethionine.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service