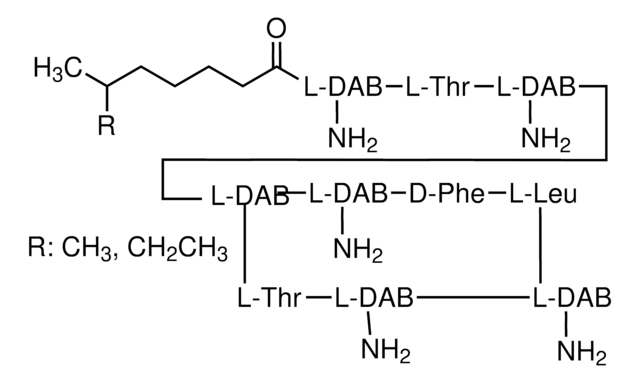
A7926
Azlocillin sodium salt
Synonym(s):
D-α-([Imidazolidin-2-on-1-yl]carbonylamino)benzylpenicillin
About This Item
Recommended Products
form
powder
Quality Level
antibiotic activity spectrum
Gram-negative bacteria
Gram-positive bacteria
mode of action
cell wall synthesis | interferes
storage temp.
room temp
SMILES string
[Na+].CC1(C)SC2[C@H](NC(=O)[C@H](NC(=O)N3CCNC3=O)c4ccccc4)C(=O)N2[C@H]1C([O-])=O
InChI
1S/C20H23N5O6S.Na/c1-20(2)13(17(28)29)25-15(27)12(16(25)32-20)22-14(26)11(10-6-4-3-5-7-10)23-19(31)24-9-8-21-18(24)30;/h3-7,11-13,16H,8-9H2,1-2H3,(H,21,30)(H,22,26)(H,23,31)(H,28,29);/q;+1/p-1/t11-,12-,13+,16-;/m1./s1
InChI key
UVOCNBWUHNCKJM-XFAPPKAWSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
application
Biochem/physiol Actions
Other Notes
signalword
Danger
hcodes
pcodes
Hazard Classifications
Resp. Sens. 1 - Skin Sens. 1
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service