E9750
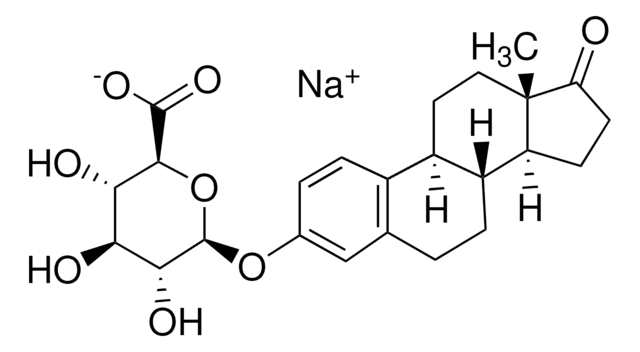
Estrone
≥99% (HPLC), powder, estrogen receptor agonist
Synonym(s):
1,3,5(10)-Estratrien-3-ol-17-one, 3-Hydroxy-1,3,5(10)-estratrien-17-one, Folliculin
About This Item
Recommended Products
Product Name
Estrone, ≥99%
biological source
synthetic (organic)
Quality Level
sterility
non-sterile
assay
≥99%
form
powder
mp
258-260 °C (lit.)
solubility
dioxane: 50 mg/mL, clear, colorless to very faintly yellow
shipped in
ambient
storage temp.
room temp
Looking for similar products? Visit Product Comparison Guide
General description
Application
- as medium supplement for hormone based degranulation studies of natural killer cells[4]
- as an endocrine disrupting compound for screening bacterial biosensor in toxic water.[5]
- as medium component for monitoring fatty acid synthase (FASN) activity in breast adenocarcinoma cell lines[6]
Biochem/physiol Actions
Features and Benefits
related product
signalword
Danger
hcodes
Hazard Classifications
Carc. 2 - Lact. - Repr. 1A
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Discover Bioactive Small Molecules for ADME/Tox
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service