F0896
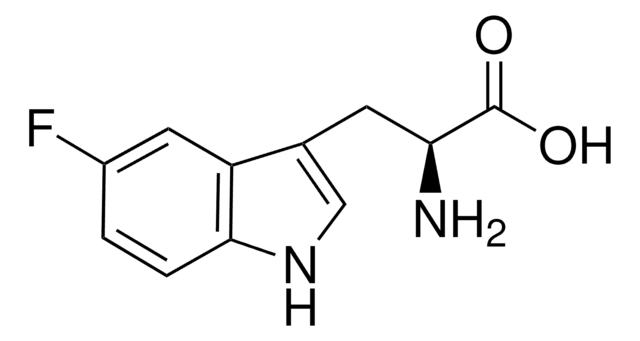
5-Fluoro-DL-tryptophan
≥95% (HPLC)
Synonym(s):
5-fluoro-tryptophan
Select a Size
Select a Size
About This Item
Recommended Products
Product Name
5-Fluoro-DL-tryptophan, powder or crystals
assay
≥95% (HPLC)
Quality Level
form
powder or crystals
color
slightly off-white to brown
application(s)
detection
peptide synthesis
storage temp.
2-8°C
SMILES string
NC(Cc1c[nH]c2ccc(F)cc12)C(O)=O
InChI
1S/C11H11FN2O2/c12-7-1-2-10-8(4-7)6(5-14-10)3-9(13)11(15)16/h1-2,4-5,9,14H,3,13H2,(H,15,16)
InChI key
INPQIVHQSQUEAJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Determination of the 19F NMR chemical shielding tensor and crystal structure of 5-fluoro-dl-tryptophan.: This study provides a detailed analysis of the 19F NMR chemical shielding tensor and the crystal structure of 5-Fluoro-dl-tryptophan, offering valuable insights into its electronic environment and molecular interactions. Such detailed structural characterization is crucial for the development of fluorinated drugs and biomolecules, enhancing our understanding of their interactions and functionalities (Sykes BD et al., 2007).
Biochem/physiol Actions
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service