G115000
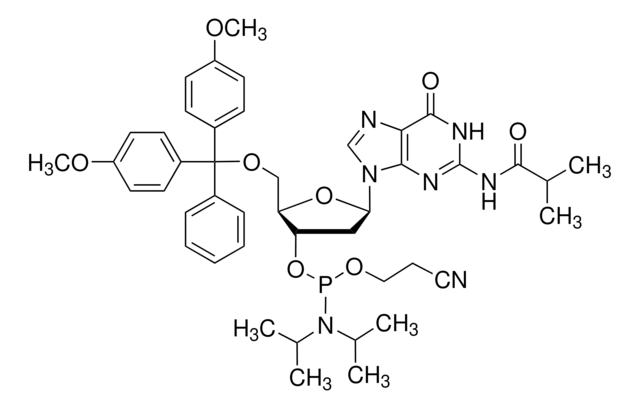
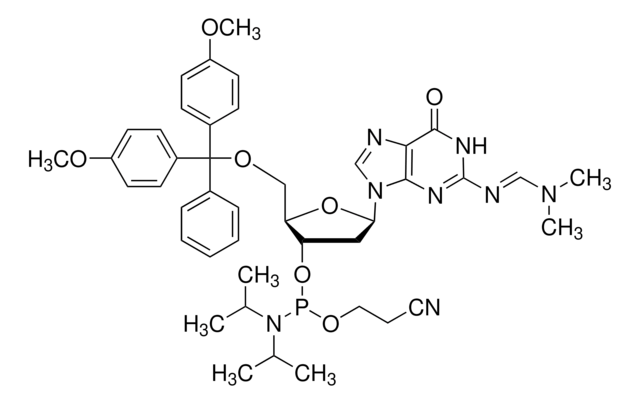
DMT-dG(dmf) Phosphoramidite
≥99.0% (reversed phase HPLC)
Synonym(s):
DMT-dG(dmf) Amidite, N-[(dimethylamino)methylene]-5′-O-[bis(4-methoxyphenyl)phenylmethyl]-2′-deoxyguanosine, 3′-[2-cyanoethyl N,N-bis(1-methylethyl)phosphoramidite], N2-Dimethylformamidine-5′-O-(4,4′-dimethoxytrityl)-2′-deoxyguanosine-3′-O-[O-(2-cyanoethyl)-N,N′-diisopropylphosphoramidite]
About This Item
Recommended Products
type
for DNA synthesis
Quality Level
product line
Proligo Reagents
assay
≥99% (31P-NMR)
≥99.0% (reversed phase HPLC)
form
powder
mol wt
824.90 g/mol
technique(s)
oligo synthesis: suitable
color
white to off-white
λ
conforms (UV/VIS Identity)
nucleoside profile
base: deoxyguanosine
base protecting group: DMF
2' protecting group: none
5' protecting group: DMT
deprotection: fast
storage temp.
-10 to -25°C
SMILES string
COc1ccc(cc1)C(OC[C@H]2O[C@H](C[C@@H]2OP(OCCC#N)N(C(C)C)C(C)C)n3cnc4C(=O)NC(\N=C/N(C)C)=Nc34)(c5ccccc5)c6ccc(OC)cc6
InChI
1S/C43H53N8O7P/c1-29(2)51(30(3)4)59(56-24-12-23-44)58-36-25-38(50-28-45-39-40(50)47-42(48-41(39)52)46-27-49(5)6)57-37(36)26-55-43(31-13-10-9-11-14-31,32-15-19-34(53-7)20-16-32)33-17-21-35(54-8)22-18-33/h9-11,13-22,27-30,36-38H,12,24-26H2,1-8H3,(H,47,48,52)/b46-27-/t36-,37+,38+,59?/m0/s1
InChI key
YRQAXTCBMPFGAN-UJASEYITSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Features and Benefits
- dG(dmf) is deprotected faster than the conventional dG(ib): the deprotection time in concentrated ammonia is reduced to 2 hours at 55 °C or 1 hour at 65 °C
- The dG(dmf)-monomer is especially suitable for G-rich sequences: incomplete deprotection is greatly reduced in comparison with the conventional dG(ib)-monomer
- dG(dmf)-phosphoramidite is as stable in solution as the standard dA(bz)-, dC(bz)- and dT-phosphoramidites
- dG(dmf)-phosphoramidite can directly substitute for dG(ib)-phosphoramidite
- No change is required in the reagents commonly used for DNA synthesis (Except a low concentration iodine oxidizer i.e., 0.02 M in iodine, should be employed)
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service