G1377
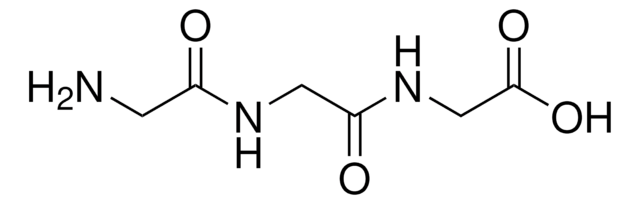
Gly-Gly-Gly
≥98% (TLC)
Synonym(s):
Glycyl-glycyl-glycine, Triglycine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NH2CH2CONHCH2CONHCH2COOH
CAS Number:
Molecular Weight:
189.17
Beilstein/REAXYS Number:
1711130
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
Gly-Gly-Gly,
assay
≥98% (TLC)
Quality Level
form
powder
color
white
application(s)
peptide synthesis
SMILES string
NCC(=O)NCC(=O)NCC(O)=O
InChI
1S/C6H11N3O4/c7-1-4(10)8-2-5(11)9-3-6(12)13/h1-3,7H2,(H,8,10)(H,9,11)(H,12,13)
InChI key
XKUKSGPZAADMRA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Amino Acid Sequence
Gly-Gly-Gly
Application
Triglycine (Gly-Gly-Gly) is used as a model peptide for studies of physicochemical parameters and molecular associations of small peptides. Triglycine is used as a copper chelator.
Biochem/physiol Actions
Substrate for reproducible serum protein measurements by the biuret reaction and for the assay of aminotripeptidases.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Blake E Ziegler et al.
The journal of physical chemistry. A, 114(44), 11953-11963 (2010-10-26)
Hydrogen/deuterium exchange reactions involving protonated triglycine and deuterated ammonia (ND(3)) have been examined in the gas phase using a Fourier transform ion cyclotron resonance (FT-ICR) mass spectrometer. Ab initio and density functional theory (DFT) calculations have been carried out to
S J Woltman et al.
Analytical chemistry, 67(3), 541-551 (1995-02-01)
The reversible electrochemistry of the Cu(II)/Cu(III) couple was investigated for the copper(II) complexes of triglycine (G3), tetraglycine (G4), and pentaglycine (G5) in alkaline solution using a rotating ring-disk electrode (RRDE). The study was motivated by the need to elucidate electrochemical
Chi-Kit Siu et al.
Journal of the American Society for Mass Spectrometry, 20(6), 996-1005 (2009-03-04)
Fragmentations of tautomers of the alpha-centered radical triglycine radical cation, [GGG(*)](+), [GG(*)G](+), and [G(*)GG](+), are charge-driven, giving b-type ions; these are processes that are facilitated by a mobile proton, as in the fragmentation of protonated triglycine (Rodriquez, C. F. et
Jiyun Shi et al.
Bioconjugate chemistry, 20(4), 750-759 (2009-03-27)
Radiolabeled cyclic RGD (Arg-Gly-Asp) peptides represent a new class of radiotracers with potential for early tumor detection and noninvasive monitoring of tumor metastasis and therapeutic response in cancer patients. This article describes the synthesis of two cyclic RGD peptide dimer
Sudipta Chakraborty et al.
Bioconjugate chemistry, 21(5), 969-978 (2010-04-15)
This report presents the synthesis and evaluation of (111)In(DOTA-6G-RGD(4)) (DOTA = 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetracetic acid; 6G-RGD(4) = E{G(3)-E[G(3)-c(RGDfK)](2)}(2) and G(3) = Gly-Gly-Gly), (111)In(DOTA-RGD(4)) (RGD(4) = E{E[c(RGDfK)](2)}(2)) and (111)In(DOTA-3G-RGD(2)) (3G-RGD(2) = G(3)-E[G(3)-c(RGDfK)](2)) as new radiotracers for imaging integrin alpha(v)beta(3)-positive tumors. The IC(50) values
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service