All Photos(2)
About This Item
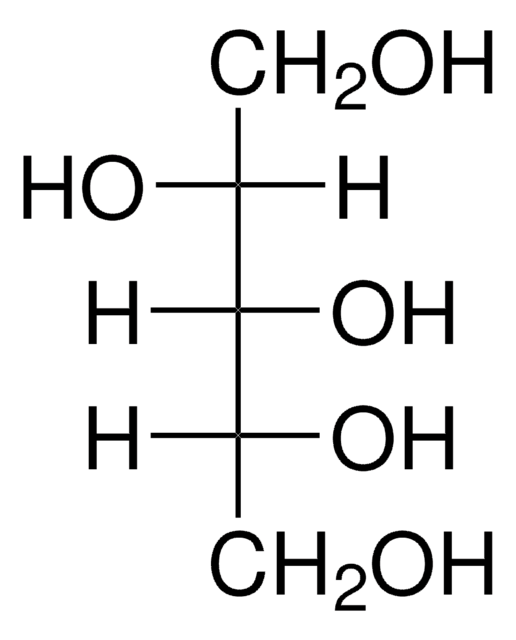
Empirical Formula (Hill Notation):
C6H14O6
CAS Number:
Molecular Weight:
182.17
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
assay
≥98% (GC)
form
solid
color
white
mp
78-80 °C (lit.)
solubility
water: 50 mg/mL, clear, colorless
Storage temp.
2-8°C
SMILES string
OC[C@H](O)[C@@H](O)[C@H](O)[C@@H](O)CO
InChI
1S/C6H14O6/c7-1-3(9)5(11)6(12)4(10)2-8/h3-12H,1-2H2/t3-,4-,5+,6+/m0/s1
Inchi Key
FBPFZTCFMRRESA-UNTFVMJOSA-N
Looking for similar products? Visit Product Comparison Guide
General description
A rare sugar alcohol (polyol).
Other Notes
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Wojciech Schönemann et al.
Bioorganic & medicinal chemistry, 18(7), 2645-2650 (2010-03-17)
A short synthesis of new beta-1-C-alkyl-1,5-dideoxy-1,5-imino-l-iditols by means of the diastereoselective addition of Grignard reagents onto a glucopyranosylamine is described. These compounds were evaluated as beta-glucocerebrosidase inhibitors and their activity was compared with that of related iminosugar derivatives in the
Constitution of the dianhydrides of sorbitol and iditol.
R MONTGOMERY et al.
Nature, 157, 372-372 (1946-03-23)
N A Williams et al.
Journal of parenteral science and technology : a publication of the Parenteral Drug Association, 47(3), 119-123 (1993-05-01)
The mechanical properties of frozen mannitol, L-iditol, dulcitol, and sorbitol solutions were measured as a function of temperature during warming (after freezing) using a thermal mechanical analyzer (TMA). The mannitol sample first underwent a contractive phase starting at 30 degrees
David M Hodgson et al.
Organic letters, 7(12), 2305-2308 (2005-06-04)
[reaction: see text] Reaction of hindered lithium amides with readily available (enantiopure) terminal epoxides gives 2-ene-1,4-diols via carbenoid dimerization of the corresponding alpha-lithiated epoxides. D-Mannitol and D-iditol were synthesized using this method in three steps from (S)-tritylglycidyl ether.
Hexitol anhydrides; 1,4,3,6-dianhydro-L-iditol and the structures of isomannide and isosorbide.
H G FLETCHER et al.
Journal of the American Chemical Society, 68, 939-941 (1946-06-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service