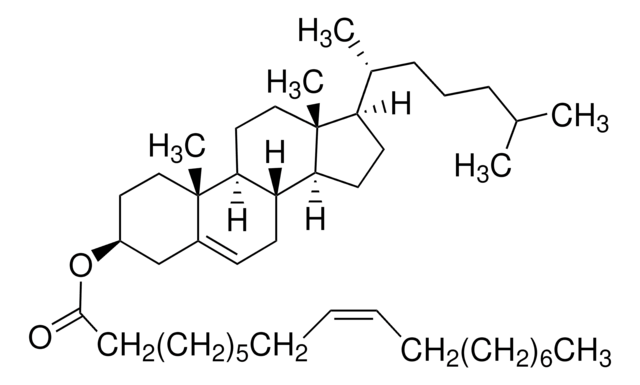
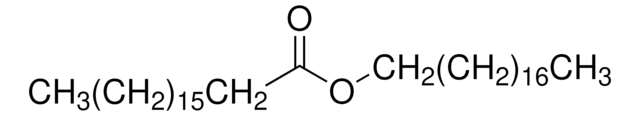
O3380
Oleyl oleate
≥99%
Synonym(s):
Oleyl oleate, Oleic acid oleyl ester
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C36H68O2
CAS Number:
Molecular Weight:
532.92
EC Number:
MDL number:
UNSPSC Code:
12352211
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Quality Level
assay
≥99%
form
liquid
functional group
ester
oleic acid
lipid type
unsaturated FAs
shipped in
ambient
storage temp.
−20°C
SMILES string
CCCCCCCC\C=C/CCCCCCCCOC(=O)CCCCCCC\C=C/CCCCCCCC
InChI
1S/C36H68O2/c1-3-5-7-9-11-13-15-17-19-21-23-25-27-29-31-33-35-38-36(37)34-32-30-28-26-24-22-20-18-16-14-12-10-8-6-4-2/h17-20H,3-16,21-35H2,1-2H3/b19-17-,20-18-
InChI key
BARWIPMJPCRCTP-CLFAGFIQSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Group 13 Lewis acid catalyzed synthesis of metal oxide nanocrystals via hydroxide transmetallation.: This research utilizes oleyl oleate as a solvent to facilitate the synthesis of metal oxide nanocrystals, showcasing its effectiveness in nanoparticle production and potential applications in various electronic and optical devices (Gibson et al., 2021).
- High-level accumulation of oleyl oleate in plant seed oil by abundant supply of oleic acid substrates to efficient wax ester synthesis enzymes.: This article details the genetic engineering approaches to enhance oleyl oleate content in plant oils, aiming at industrial applications such as biofuels and biolubricants (Yu et al., 2018).
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
452.3 °F
flash_point_c
233.5 °C
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J Zhang et al.
Chinese journal of biotechnology, 11(4), 243-251 (1995-01-01)
The effect of 14 different lipases on oleyl oleate synthesis were compared. The lipase from Candida sp.1619 had the highest esterification activity. The lipase was immobilized by adsorbing it on celite with 0.1 (w/w) coconut oil, Tween 80, and 1%
T Rostrup-Nielsen et al.
Journal of chemical technology and biotechnology (Oxford, Oxfordshire : 1986), 48(4), 467-482 (1990-01-01)
The kinetics of enzymatic hydrolysis of oleyl oleate in the boundary layer between stagnant organic and aqueous phases was studied using a commercial lipase preparation which was dissolved in the aqueous phase. Three aspects of the reaction were investigated. (1)
José Aracil et al.
Journal of biotechnology, 124(1), 213-223 (2006-02-21)
A comparative study of the oleyl oleate production using conventional and enzymatic catalysts has been carried out. The present paper describes the flow diagrams for these processes and compares operation conditions for batch reaction and the downstream proceedings. In addition
K Ichihara et al.
Lipids, 31(5), 535-539 (1996-05-01)
An improved rapid procedure to determine the fatty acid composition of glycerolipids is described. The procedure includes KOH-catalyzed transesterification and high-speed gas chromatography. Glycerolipids (20-40 mg) were mixed with 2 mL of hexane and 0.2 mL of 2 M methanolic
Shi-You Jiang et al.
Nature communications, 9(1), 5138-5138 (2018-12-05)
Statins are inhibitors of HMG-CoA reductase, the rate-limiting enzyme of cholesterol biosynthesis, and have been clinically used to treat cardiovascular disease. However, a paradoxical increase of reductase protein following statin treatment may attenuate the effect and increase the side effects.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service