P3886
Poly-L-proline
suitable for ligand binding assays, Mol wt >30,000
About This Item
Recommended Products
product name
Poly-L-proline, mol wt >30,000
form
powder
Quality Level
mol wt
>30,000
technique(s)
ligand binding assay: suitable
color
white to light yellow
storage temp.
−20°C
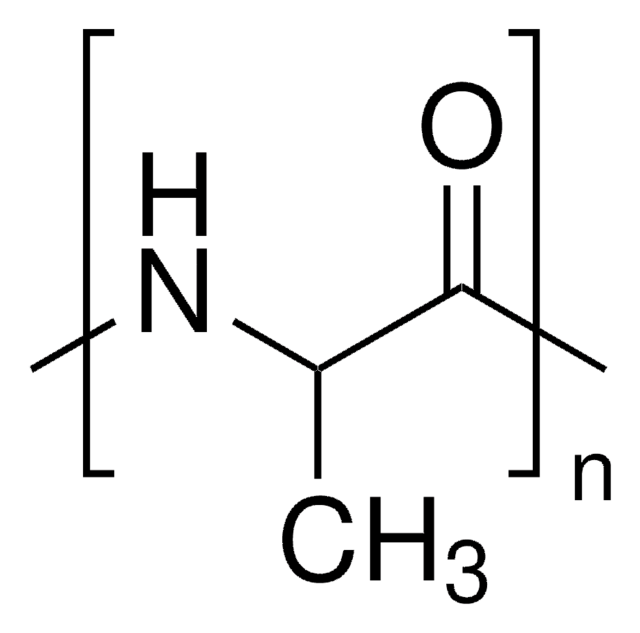
SMILES string
OC(=O)C1CCCN1
InChI
1S/C5H9NO2/c7-5(8)4-2-1-3-6-4/h4,6H,1-3H2,(H,7,8)
InChI key
ONIBWKKTOPOVIA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Analysis Note
Other Notes
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Humankind has utilized protein materials throughout its existence, starting with the use of materials such as wool and silk for warmth and protection from the elements and continuing with the use of recombinant DNA techniques to synthesize proteins with unique and useful properties.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service