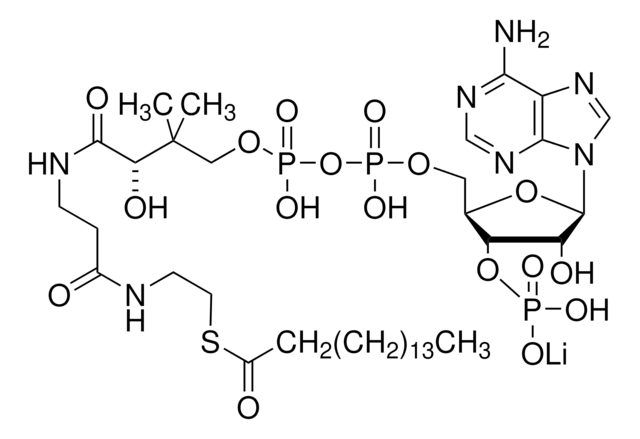
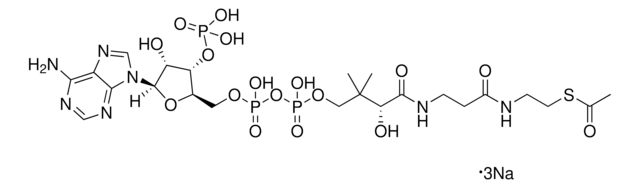
SRP6103
MDH1 human
recombinant, expressed in E. coli, ≥95% (SDS-PAGE)
Synonym(s):
MDH-s, MDHA, MOR2, Malate dehydrogenase cytoplasmic
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
biological source
human
recombinant
expressed in E. coli
assay
≥95% (SDS-PAGE)
form
liquid
mol wt
37.4 kDa (342 aa, 1-334 aa + CT His Tag)
packaging
pkg of 100 μg
technique(s)
activity assay: suitable
NCBI accession no.
shipped in
dry ice
storage temp.
−70°C
Gene Information
human ... MDH1(4190)
General description
MDH1 (malate dehydrogenase 1) is cytoplasmic protein belonging to the 2-hydroxy acid dehydrogenases protein family. Eukaryotes contain two forms of MDH proteins depending upon their subcellular localization, MDH1 and MDH2. MDH2 is present in the mitochondrial matrix and MDH1 is present in the cytoplasm. This gene is localized to human chromosome 2, and is composed of nine exons and eight introns.
Application
MDH1 (malate dehydrogenase 1) human has been used for measuring MDH1 enzyme activity.
Biochem/physiol Actions
MDH1 (malate dehydrogenase 1) is responsible for reversibly converting malate to oxaloacetate in cytoplasm. It participates in metabolism where it transports NADH equivalents and metabolites across the mitochondrial membrane, and regulates the TCA (tricarboxylic acid) cycle pool size. MDH1 is involved in malate/aspartate shuttle, which is involved in maintaining the balance of nitrogen, oxaloacetate, and the α-ketoglutarate intermediate shuttling between the cytosol and mitochondrial matrix. It is thought to function as an important checkpoint for energy balance between mitochondria and cytoplasm. MDH1 acts as a regulator of p53-dependent apoptosis in response to glucose deprivation, indicating that synergism between energy metabolism and p53 transactivation is linked with cell metabolic state and hence, determination of cell death.
Physical form
1 mg/mL solution in 20 mM Tris-HCl (pH 8.0) containing 10% glycerol.
Preparation Note
Centrifuge the vial prior to opening.
Other Notes
MSEPIRVLVT GAAGQIAYSL LYSIGNGSVF GKDQPIILVL LDITPMMGVL DGVLMELQDC ALPLLKDVIA TDKEDVAFKD LDVAILVGSM PRREGMERKD LLKANVKIFK SQGAALDKYA KKSVKVIVVG NPANTNCLTA SKSAPSIPKE NFSCLTRLDH NRAKAQIALK LGVTANDVKN VIIWGNHSST QYPDVNHAKV KLQGKEVGVY EALKDDSWLK GEFVTTVQQR GAAVIKARKL SSAMSAAKAI CDHVRDIWFG TPEGEFVSMG VISDGNSYGV PDDLLYSFPV VIKNKTWKFV EGLPINDFSR EKMDLTAKEL TEEKESAFEF LSSALEHHHH HH
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A nucleocytoplasmic malate dehydrogenase regulates p53 transcriptional activity in response to metabolic stress.
Lee SM et al
Cell Death and Differentiation, 16(5), 738-748 (2009)
Ultra high throughput sequencing excludes MDH1 as candidate gene for RP28-linked retinitis pigmentosa.
Rio Frio T et al
Molecular Vision, 15, 2627-2633 (2009)
Tomohiro Yoshida et al.
Biochemical and biophysical research communications, 522(3), 633-638 (2019-12-04)
Metabolic programs are rewired in cancer cells to support survival and tumor growth. Among these, recent studies have demonstrated that glutamate-oxaloacetate transaminase 1 (GOT1) plays key roles in maintaining redox homeostasis and proliferation of pancreatic ductal adenocarcinomas (PDA). This suggests
Edouard Mullarky et al.
Proceedings of the National Academy of Sciences of the United States of America, 113(7), 1778-1783 (2016-02-03)
Cancer cells reprogram their metabolism to promote growth and proliferation. The genetic evidence pointing to the importance of the amino acid serine in tumorigenesis is striking. The gene encoding the enzyme 3-phosphoglycerate dehydrogenase (PHGDH), which catalyzes the first committed step
Eun Young Kim et al.
Journal of lipid research, 53(9), 1864-1876 (2012-06-14)
Acetylation is one of the most crucial post-translational modifications that affect protein function. Protein lysine acetylation is catalyzed by acetyltransferases, and acetyl-CoA functions as the source of the acetyl group. Additionally, acetyl-CoA plays critical roles in maintaining the balance between
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service