1466674
USP
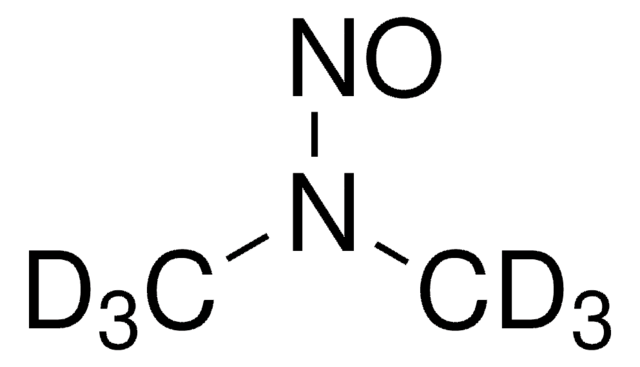
N-Nitrosodimethylamine (NDMA)
United States Pharmacopeia (USP) Reference Standard
Synonym(s):
N-Methyl-N-nitrosomethanamine, NDMA
About This Item
Recommended Products
packaging
pkg of 1 mg
manufacturer/tradename
USP
application(s)
pharmaceutical (small molecules)
format
single component solution (solution in methanol)
storage temp.
−20°C
SMILES string
N(N=O)(C)C
InChI
1S/C2H6N2O/c1-4(2)3-5/h1-2H3
InChI key
UMFJAHHVKNCGLG-UHFFFAOYSA-N
General description
Application
It is also used to prepare standard, standard stock, nitrosamine RS stock, nitrosamine standards stock solution mixture, and sensitivity stock solutions to determine NDMA impurity in drug substances and drug products(valsartan, irbesartan, and losartan potassium etc.) by chromatography method according to the general chapter 〈1469〉 of United States Pharmacopeia.
Analysis Note
Other Notes
signalword
Danger
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Carc. 1B - Flam. Liq. 2 - STOT SE 1
target_organs
Eyes,Central nervous system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
49.5 °F
flash_point_c
9.7 °C
Choose from one of the most recent versions:
Certificates of Analysis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
An overview of nitrosamine impurity testing, worldwide regulations, and key considerations in filter selection for sample preparation and analysis.
This application note describes the LC-MS-based quantitative analysis of known nitrosamine impurities following procedures 1 and 3 as given in USP general chapter <1469>.
GC-MS method detects nitrosamines in Valsartan tablets, meeting US FDA guidelines for pharmaceutical quality control.
Related Content
Order from a broad range of highly characterized primary reference standard materials to use with USP-NF monographs for the testing of drug substances & dosage forms, pharmaceutical excipients, food ingredients and dietary supplements.
Accurately detect and quantify trace nitrosamines (NDMA, NDEA, NEIPA, NDIPA, NDBA, etc.) in pharmaceutical drugs using our complete product portfolio and application guides for LC-MS and GC-MS. Order high-quality reference standards, columns, filters & more.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service