1673001
USP
Travoprost
United States Pharmacopeia (USP) Reference Standard
Synonym(s):
Travoprost solution
About This Item
Recommended Products
grade
pharmaceutical primary standard
API family
travoprost
manufacturer/tradename
USP
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
−20°C
SMILES string
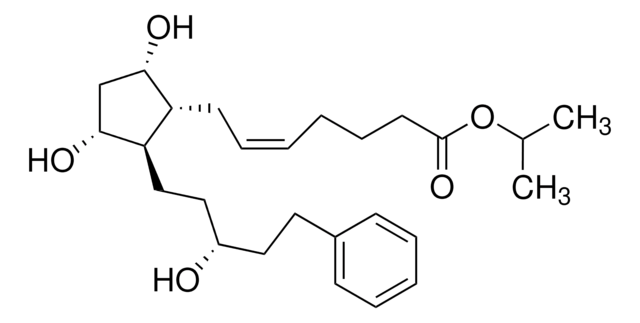
FC(F)(F)c1cc(ccc1)OC[C@H](O)\C=C\[C@@H]2[C@H]([C@H](C[C@H]2O)O)C\C=C/CCCC(=O)OC(C)C
InChI
1S/C26H35F3O6/c1-17(2)35-25(33)11-6-4-3-5-10-21-22(24(32)15-23(21)31)13-12-19(30)16-34-20-9-7-8-18(14-20)26(27,28)29/h3,5,7-9,12-14,17,19,21-24,30-32H,4,6,10-11,15-16H2,1-2H3/b5-3-,13-12+/t19-,21-,22-,23+,24-/m1/s1
InChI key
MKPLKVHSHYCHOC-AHTXBMBWSA-N
Looking for similar products? Visit Product Comparison Guide
General description
For further information and support please go to the website of the issuing Pharmacopoeia.
Application
- Innovative eye drop applications: Travoprost has been incorporated into engineered lipoprotein nanoparticles, showcasing a novel method for drug delivery aimed at enhancing therapeutic efficacy in ocular treatments, particularly for conditions like glaucoma and ocular hypertension (Fukuda et al., 2024).
- Prostaglandin analogs in glaucoma treatment: The effectiveness and safety of benzalkonium chloride-preserved travoprost eye drops have been critically evaluated, providing insights into their impact on ocular health, particularly on conjunctival goblet cells, which are vital for maintaining eye surface health in glaucoma patients (Nagstrup, 2023).
- Travoprost liquid nanocrystals: Research into travoprost liquid nanocrystals has demonstrated their potential as a significant advancement in glaucoma therapy, offering improved delivery mechanisms and potentially greater efficacy in reducing intraocular pressure (El-Gendy et al., 2023).
- Cerebrospinal fluid reabsorption: Studies on prostaglandin analog effects on cerebrospinal fluid reabsorption via nasal mucosa reveal new roles for travoprost in physiological processes beyond ocular applications, indicating potential new therapeutic pathways (Pedler et al., 2021).
Analysis Note
Other Notes
signalword
Danger
hcodes
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2
Storage Class
3 - Flammable liquids
wgk_germany
WGK 2
flash_point_f
55.4 °F
flash_point_c
13 °C
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service