Determination of Headspace Volatiles in Distilled Spirits Using SPME Coupled to GC
Robert E. Shirey, Michael D. Buchanan, Daniel Vitkuske
Reporter US Volume 34.1
A beverage manufacturer may be interested in the overall composition of their product, or may need to determine the levels of alcohol, caffeine, nutraceuticals, antioxidants, vitamins, sweeteners, aroma, off-flavors, and/or impurities. Sample preparation of a beverage is often simple, and can usually be accomplished with direct injection, headspace, solid phase microextraction (SPME), or solid phase extraction (SPE). For distilled spirits, the different aroma volatiles present can greatly influence the organoleptic properties of the beverage. In this work, we show that SPME coupled with gas chromatography (GC) is an effective technique for monitoring the volatiles present in the headspace over different distilled spirits.
Experimental
Aroma involves the sense of smell, so SPME sampling occurred in the headspace over the spirit samples. A ratio of 4 mL of sample in a 10 mL vial provided an adequate volume for the headspace to evolve, plus enough physical space for the SPME fiber to be exposed. For the fiber, a 50/30 μm divinylbenzene/Carboxen®/polydimethylsiloxane coating on a 1 cm long StableFlex™ core was used to maximize the variety of compound types extracted. Each sorbent is more selective towards a different class of compounds as follows:
- Divinylbenzene (DVB) for aromatic compounds
- Carboxen for small-sized molecules
- Polydimethylsiloxane (PDMS) for hydrocarbons
A flexible StableFlex core is an improvement over a more rigid fused silica core. In addition to being a less breakable fiber, the sorbent coating will partially bond to the StableFlex, resulting in a more stable coating. For the GC analysis, a VOCOL® capillary GC column was used. Its intermediate polar nature enables strong dispersive (van der Waals forces) analyte-stationary phase interactions, along with π-π, dipole-dipole, and dipole-induced dipole interactions. The ‘thick’ 1.50 μm film is necessary to provide focusing and increased retention of volatile analytes.
Slight heat, agitation of the vial, stirring, and the additional of salt are common ways to help drive volatiles into the headspace, which will increase sensitivity. The autosampler was programmed with a simple extraction (40 °C for 15 minutes with agitation). Replicates with salt (sodium chloride) added at 15% (w/v), and without salt, were extracted and analyzed for the first sample (a Kentucky bourbon). Similar chromatographic results were observed for both replicates, so the addition of salt was dropped to keep the extraction simple.
Nine spirit samples were tested using the same SPME-GC conditions (listed in Figure 1). Included were 1 gin, 1 Tennessee whiskey, 2 Kentucky bourbons, 1 Canadian whiskey, 1 Irish whiskey, 2 Scotch whiskeys, and 1 bourbon liqueur. The resulting chromatograms from three different spirit types are included in this article.
Results and Discussion
As expected, a large ethanol peak was found in each sample. The column and conditions used produced a sharp ethanol peak shape. Therefore, it did not interfere chromatographically with the volatile compounds to be identified. The other volatile compounds which were identified in these samples are listed in Table 1, along with their corresponding aroma properties.1
As shown in Figure 1, monoterpenes and diterpenes were abundant in the gin headspace. These terpenes contribute to the pine smell the sample exhibited. As shown in Figure 2, the scotch whiskey headspace was dominated by ethyl decanoate and ethyl octanoate, and moderate levels of ethyl dodecanoate and 3-methyl-butanol. It smelled of caramel, malty, and earthy aromas. As shown in Figure 3, the bourbon liqueur headspace contained an abundance of limonene, ethyl hexanoate, and methylbutyl isobutyrate. These compounds impart the sweet orange, cherry, and mint aromas that this sample displayed.
An argument for using SPME for headspace analysis can be made because this technique shows typically higher sensitivities than classical static headspace applications. This is due to the introduction of a second equilibrium between the SPME fiber and the headspace.
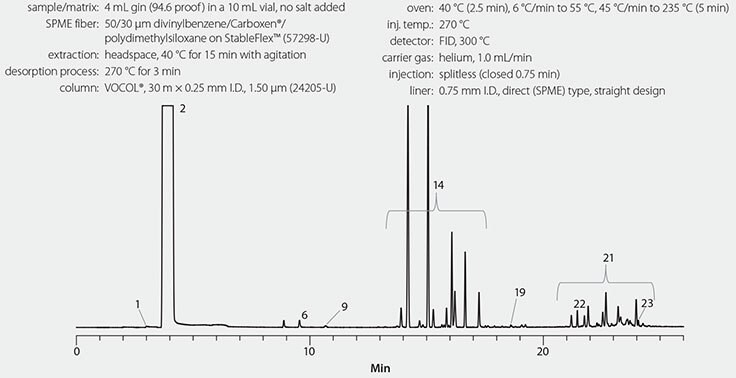
Figure 1.Gin

Figure 2.Scotch (18 Year Old)
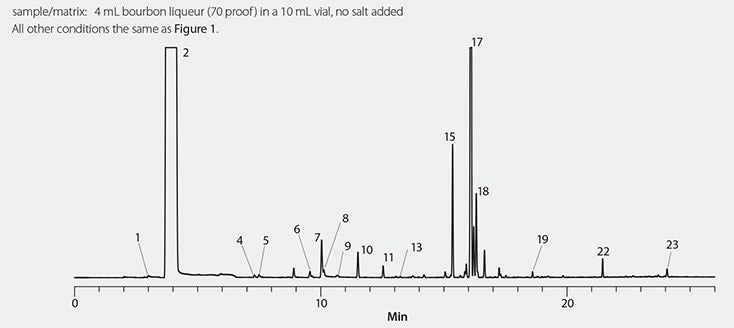
Figure 3.Bourbon Liqueur
Conclusion
SPME (over 25 years old) and GC (over 60 years old) are both mature analytical techniques. Their versatility is demonstrated by their use in multiple industries for a wide variety of applications. One such use is aroma analysis of beverages, such as spirits. In this article, SPME-GC was successfully used to identify many volatile compounds present in the headspace over several spirits.
References
To continue reading please sign in or create an account.
Don't Have An Account?