Removal of Albumin Using Blue Sepharose® Chromatography Media
Blue Sepharose® 6 Fast Flow or prepacked HiTrap™ Blue HP 1 mL and 5 mL columns (Figure 4.2) can be used to remove albumin either before or after other purification steps (Table 4.1). The albumin binds in a nonspecific manner by electrostatic and/or hydrophobic interactions with the aromatic anionic ligand, Cibacron Blue F3G-A, coupled to Sepharose®.
Figure 4.2.Prepacked with Blue Sepharose® High Performance, HiTrap™ Blue HP columns offer fast and simple removal of albumin by affinity chromatography.
Use HiTrap™ Blue HP 1 mL or 5 mL columns to remove host albumin from mammalian expression systems or when the sample is known to contain high levels of albumin that might mask the UV absorption of other protein peaks.
Do not use Blue Sepharose® chromatography media if the immunoglobulin or other target molecule has a hydrophobicity similar to that of albumin.
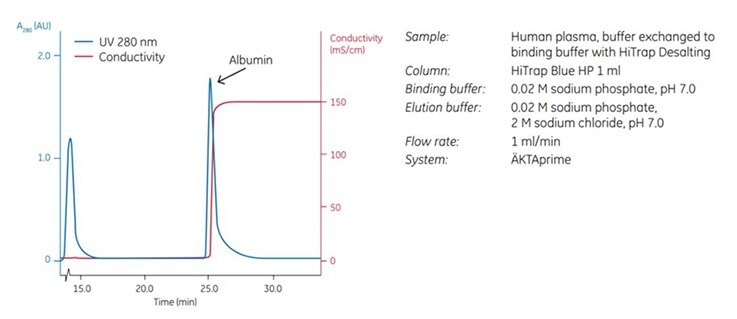
Figure 4.3.Effective removal of albumin from human plasma using a HiTrap™ Blue HP 1 mL column.
Protocol for Removing Albumin using HiTrap™ Blue HP Columns
Buffer preparation
Binding buffer: 20 mM sodium phosphate, pH 7.0, or 50 mM potassium hydrogen phosphate (KH2PO4), pH 7.0
Elution buffer: 0.02 M sodium phosphate, 2 M sodium chloride, pH 7.0, or 0.05 M potassium hydrogen phosphate, 1.5 M potassium chloride, pH 7.0
Albumin removal
- Fill the syringe or pump tubing with distilled water. Remove the stopper and connect the column to the syringe (use the connector supplied), laboratory pump, or chromatography system “drop to drop” to avoid introducing air into the system.
- Remove the snap-off end at the column outlet.
- Wash out the ethanol with 3 to 5 column volumes of distilled water.
- Equilibrate the column with at least 5 column volumes of binding buffer. Recommended flow rates are 1 mL/min (1 mL column) and 5 mL/min (5 mL column).
- Apply the sample using a syringe fitted to the Luer connector or by pumping it onto the column. For optimal results, use a flow rate of 0.2 to 1 mL/min (1 mL column) and 0.5 to 5 mL/min (5 mL column) during sample application*.
- Wash with binding buffer (generally at least 5 to 10 column volumes) until the absorbance reaches a steady baseline or no material remains in the effluent. Maintain a flow rate of 1 to 2 mL/min (1 mL column) and 5 to 10 mL/min (5 mL column) for washing.
- Elute with elution buffer using a one-step or linear gradient. For step elution, 5 column volumes is usually sufficient. For linear gradient elution, 10 to 20 column volumes are usually sufficient. Maintain a flow rate of 1 to 2 mL/min (1 mL column) and 5 to 10 mL/min (5 mL column) for elution.
- After elution, regenerate the column by washing it with 3 to 5 column volumes of binding buffer. The column is now ready for a new purification.
* 1 mL/min corresponds to approximately 30 drops/min when using a syringe with a 1 mL HiTrap™ column; 5 mL/min corresponds to approximately 120 drops/min when using a 5 mL HiTrap™ column.
Storage
Store in 20% ethanol at 2 °C to 8 °C.
To continue reading please sign in or create an account.
Don't Have An Account?