Gas Chromatography (GC) Column Selection Guide
Achieve Optimal Method Performance
Section Overview
- The History of Supelco and the Capillary Column
- How to Choose a Capillary Column
- Additional Reading
- Column Phase Selection by Industry
- Column Phase Selection by Application
- Capillary Columns by Phase
- Traditional Phases: GC Column Polarity Scale
- Traditional Phases: Non-Polar
- Traditional Phases: Intermediate Polar
- Traditional Phases: Polar
- Traditional Phases: Highly Polar
- Traditional Phases: Extremely Polar
- Chiral Phases
- PLOT Columns SCOT Columns
- Catalog Numbers

The History of Supelco and the Capillary Column
Supelco began in 1966 in a tiny garage in a small central Pennsylvania (USA) town manufacturing packed gas chromatography (GC) columns. By 1977, glass capillary GC columns were being manufactured and in 1982, production began on fused silica capillary GC columns. In 1983, the first special purpose fused silica capillary GC column was introduced. Since then, an impressive list of special purpose fused silica capillary GC columns has followed. We test every capillary column we manufacture according to strict quality assurance processes, and guarantee satisfactory performance.
Technical Service chemists are a valuable resource for providing guidance with the selection and use of capillary columns. Technical Service can be reached at 800-359-3041 (USA and Canada only), 814-359-3041, here.
How to Choose a Capillary Column
An optimized chromatographic separation begins with the column. The selection of the proper capillary column for any application should be based on four significant factors: stationary phase, column I.D., film thickness, and column length. The practical effects of these factors on the performance of the column are discussed briefly in this section, in order of importance. Note that this information is general. Specific situations may warrant exceptions to these guidelines.
Step 1 – Stationary Phase
Choosing a stationary phase is the most important step in selecting a column. A stationary phase is the film coated on the inner wall of a capillary column, and should be selected based on the application to be performed. The differences in the chemical and physical properties of injected organic compounds and their interactions with the stationary phase are the basis of the separation process. When the strength of the analyte-phase interactions differs significantly for two compounds, one is retained longer than the other. How long they are retained in the column (retention time) is a measure of these analyte-phase interactions.
Changing the chemical features of the stationary phase alters its physical properties. Two compounds that co-elute (do not separate) on a particular stationary phase might separate on another phase of a different chemistry, if the difference in the analyte-phase interactions is significant. This is the reason for providing a wide variety of capillary column phases. Each phase provides a specific combination of interactions for each chemical class of analytes.
Established Applications
Gas chromatography, first established in the 1950’s, is a mature analytical technique with many established applications. Therefore, it is probable that literature, such as written methodology or journals, exists stating which stationary phases have successfully been used for a given application. Additionally, column manufacturers routinely publish phase selection charts. Charts like these are conveniently arranged by industry to simplify the process of selecting the proper phase. First, find the chart that matches your industry or area of interest. Then, locate the application within that chart to identify a recommended column phase.
New Applications
For new applications, there is often no existing reference to provide guidance. In these ‘method development’ instances, one must have some knowledge of the chemistry of the compounds to be analyzed. Phase selection is based on the general chemical principle that “likes dissolves like.” A non-polar column is the recommended starting point for the analyses of non-polar compounds. Likewise, polar columns are usually recommended for the separation of polar compounds. The “Phase Polarity” insert (see below) describes several recommended phases for each group of compound polarities.
Phase Polarity
This is the single most important characteristic in selecting a capillary column because it dictates selectivity, or the ability of the column to separate sample components. Phase selection is based on the general chemical principle that “likes dissolves like.” A non-polar column is best for the analyses of non-polar compounds. Polar columns most effectively separate polar compounds.
Non-polar compounds are generally composed only of carbon and hydrogen atoms and contain carbon-carbon single bonds. Non-polar capillary columns separate these compounds very well. Interaction between non-polar compounds and a non-polar phase are dispersive, meaning that they are governed by van der Waals forces. These are intermolecular attractions that increase with the size of the compound. Thus, larger compounds with higher boiling points have longer retention. Elution order generally follows the boiling points of the compounds.
Polar compounds are composed primarily of carbon and hydrogen atoms, but also contain one or more atoms of bromine, chlorine, fluorine, nitrogen, oxygen, phosphorus, or sulfur. Alcohols, amines, carboxylic acids, diols, esters, ethers, ketones, and thiols are typical polar compounds analyzed by capillary GC. Intermediate polar or polar capillary columns separate these compounds well. In addition to dispersive interactions, interactions between polar compounds and the phase include dipole, π-π, and/or acid-base interactions. Separations are determined by differences in the overall effects of these interactions.
Polarizable compounds are compounds composed of carbon and hydrogen, but contain one or more double or triple carbon-carbon bonds. These compounds include alkenes, alkynes, and aromatic (benzene-ring containing) hydrocarbons. Highly polar capillary columns are generally used to separate these compounds.
Bonded/Non-Bonded Phases
Bonded phases are immobilized/chemically bonded (crosslinked) within the tubing, while non-bonded phases are simply coated on the wall. Generally a bonded phase is preferred, because it exhibits less bleed during use, can be used to higher temperatures, and, when necessary, can be rinsed with solvents to remove accumulated non-volatile materials. When a bonded phase is not available, such as for the highly polar phases, look for a stabilized phase. These phases are not as permanent as bonded phases (cannot be rinsed), but have greater thermal stability than non-bonded phases. For some applications, the only choice is a non-bonded phase.
Step 2 – Column I.D.
The current range of commercially available capillary column internal diameters enables the balancing of two factors: efficiency (number of theoretical plates) and sample capacity (amount of any one sample component that can be applied to the column without causing the desired sharp peak to overload). Optimizing one of these factors requires a sacrifice from the other. The ideal I.D. for a given application is dependent on the analytical needs.
The effects of column I.D. on efficiency and sample capacity are represented (Table 1). As shown, 0.25 mm I.D. columns provide adequate plates/meter for most applications while allowing acceptable sample capacity. Because of this compromise between efficiency and sample capacity, 0.25 mm is the most popular I.D. for capillary GC columns. Columns with a smaller or larger I.D. allow the user to optimize either efficiency or sample capacity, based on the requirements of their application.
High Efficiency: Observed chromatographically as narrow and well-resolved peaks. The efficiency of a capillary column, measured in plates (N) or plates per meter (N/m), increases as the I.D. of the column decreases. This is one of the basic principles behind Fast GC (see “Fast GC Brochure” for further details). If the sample to be analyzed contains many analytes, or has analytes that elute closely together, the most narrow I.D. capillary column that is practical should be selected. Note that very narrow bore columns, such as 0.10 or 0.18 mm I.D., may require specialized equipment, such as a GC with a pressure regulator that allows a higher column head pressure.
Sample Capacity: Increases as column I.D. increases. Wide bore columns can accommodate a larger mass of each analyte in a sample than narrow bore capillary columns. Exceeding the sample capacity of a column will result in skewed peaks and decreased resolution. Therefore, if the samples to be analyzed contain compounds at high concentrations, or represent a wide range of concentrations, then a wide bore column should be considered. If the proper I.D. is chosen, the column should allow the system to provide sufficient sensitivity for the minor components without being overloaded with the major components. The analyst must decide if the loss in efficiency resulting from using a wide bore column is problematic for their application. Note that the nature of the sample components and the polarity of the phase will affect sample capacity. Non-polar phases have higher capacities for non-polar analytes, and polar phases have higher capacities for polar analytes.
Fast GC Brochure
The brochure “Fast GC: A Practical Guide for Increasing Sample Throughput without Sacrificing Quality” contains valuable information concerning Fast GC principles that is not covered in this space. Included are practical considerations, theoretical discussions, a listing of columns in Fast GC dimensions, chromatograms, a listing of related products designed to maximize performance, plus a list of literature for additional reading.
Step 3 – Film Thickness
Most 0.25 mm I.D. columns have a 0.25 or 0.50 μm film thickness. Depending on the application, the optimal film thickness may be different.
Decreasing Film Thickness:
The benefits are sharper peaks (which may increase resolution) and reduced column bleed, both of which result in increased signal-to-noise. Additionally, the column’s maximum operating temperature will be increased. The drawbacks are increased analyte interaction with the tubing wall, and decreased analyte capacity. Decreasing film thickness also allows analytes to elute with shorter retention times and at lower temperatures, which may be desirable or undesirable, depending on the application. Thinner film columns should be used for analytes with high (>300 °C) boiling points (such as pesticides, PCBs, FAMEs, phthalate esters, and other semivolatile compounds), or for trace analyses.
Increasing Film Thickness:
The benefits are reduced analyte-tubing interaction and increased sample capacity. The drawbacks are increased peak widths (which may reduce resolution), increased column bleed, and a reduced maximum operating temperature for the column. Increasing film thickness also leads to increased analyte retention (and it may also increase resolution, specifically for compounds with low k) and increased elution temperature. Depending on the application, these last effects may be either desirable or undesirable. Thicker film columns are best suited for analytes with low boiling points (such as volatile organic compounds and gases). These types of analytes are retained longer on the thicker film, which may eliminate the need for subambient oven conditions. A thicker film will also increase capacity, thus making the column more compatible for higher concentration samples than a thinner film column.
Phase Ratio (β)
Effects of phase film thickness are interdependent with column I.D. The phase ratio, beta (β), expresses the ratio of the gas volume and the stationary phase volume in a column:
| β = | column radius (μm) 2 x film thickness (μm) |
In contrast to relative terms (“thick film” and “thin film”), β values establish a distinct ranking for columns. As a general rule, select columns by β values as follows:
β values are also useful when changing column I.D. and film thickness combinations for a particular analysis, because columns with the same phase ratio will provide very similar retention times and elution order under the same analytical conditions.
Columns With Similar β Values

SLB®-5ms, 30 m x 0.53 mm I.D., 0.50 μm (β = 265)

SLB®-5ms, 30 m x 0.25 mm I.D., 0.25 μm (β = 250)
Step 4 – Column Length
Generally a 30 m column provides the best balance of resolution, analysis time, and required column head pressure (Table 2). Specific applications may warrant a different column length.
Longer Columns: Provides greater resolution, but increases back pressure. It should be stressed that doubling column length will NOT double resolution (resolution only increases according to the square root of the column length). If resolution between a critical pair is less than 1, doubling column length will not bring it to baseline (resolution value of at least 1.5). Increasing column length to increase resolution should be considered as a last resort. A more effective approach to increasing resolution is to reduce column I.D.
Shorter Columns: When great resolution is not required, such as for screening purposes or for simple samples whose components are dissimilar in chemical nature. However, if column I.D. is decreased along with length, resolution can be maintained, or in some cases, actually increased.
Additional Reading
The following is a list of GC literature written by gas chromatography experts and researchers. Consult these references to learn more about the many facets of gas chromatography.
- Harold McNair and James Miller, “Basic Gas Chromatography” (1997), Wiley, ISBN 0-471-17261-8.
- David Grant, “Capillary Gas Chromatography” (1996), Wiley, ISBN 0-471-95377-6.
- Dean Rood, “A Practical Guide to the Care, Maintenance, and Troubleshooting of Capillary Gas Chromatographic Systems” (1991), Hüthig, ISBN 3-7785-1898-4.
- Konrad Grob, “Split and Splitless Injection in Capillary GC” (1993), Hüthig, ISBN 3-7785-2151-9.
- Konrad Grob, “On-Column Injection in Capillary Gas Chromatography” (1991), Hüthig, ISBN 3-7785-2055-5.
- William McFadden, “Techniques of Combined Gas Chromatography/Mass Spectrometry: Applications in Organic Analysis” (1988), Robert E. Krieger Publishing Company, ISBN 0-89464-280-4.
- Marvin McMaster and Christopher McMaster, “GC/MS: A Practical User’s Guide” (1998), Wiley-VCH, ISBN 0-471-24826-6.
- Janusz Pawliszyn, “Solid Phase Microextraction: Theory and Practice” (1997), Wiley-VCH, ISBN 0-471-19034-9.
Column Selection by Industry
Supelco has developed the most extensive line of special purpose columns designed for industry specific applications. These columns are manufactured to deliver high resolution, great analyte response, low bleed, and long column life, thereby allowing analysts to achieve the analytical performance they require. The easy-to-read phase selection charts on the next several pages are conveniently arranged by industry to simplify the process of selecting the proper phase. First, find the chart that matches your industry. Then, locate the application within that industry to identify a recommended phase.
The stationary phase also dictates the minimum and maximum temperatures at which a column can be used. Therefore, it is critical to ensure the selected stationary phase can withstand the temperature requirements of the GC method. Temperature limitations can be located in the capillary column phase section.
Environmental Industry

Industrial Hygiene Industry

Petroleum Industry

Biofuel Industry

Chemical Industry

Agriculture Industry

Food and Beverage Industry

Flavor and Fragrance Industry

Cosmetic and Personal Care/Cleaning Product Industry

Pharmaceutical Industry
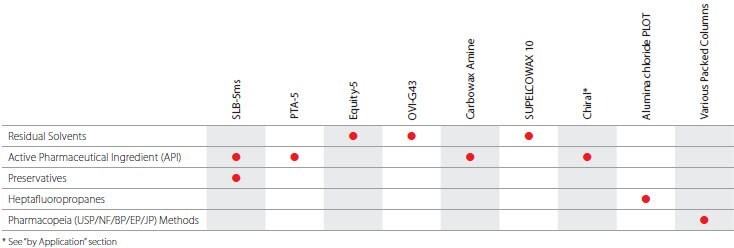
Clinical Industry

Forensic Industry

Life Science Industry

Column Selection by Application
In addition to the industry specific selection charts on the preceding pages, these easy-to-read phase selection charts highlight choices for applications that are independent of any industry. Simply locate the application to identify a recommended column phase.
The stationary phase also dictates the minimum and maximum temperatures at which a column can be used. Therefore, it is critical to ensure the selected stationary phase can withstand the temperature requirements of the GC method. Temperature limitations can be located in the capillary column phase section.
Fast GC Applications

GCxGC Applications
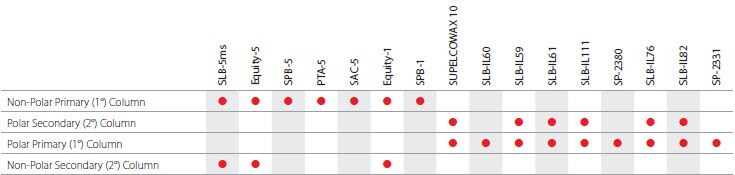
Chiral Applications
General Purpose Applications

Capillary Columns by Phase
Looking for information or specifications for a particular phase? This section includes the most popular phases and provides application, USP code, polymer and temperature limit information. Where two maximum temperatures are listed (i.e. 200/220 °C), the first is for isothermal oven analyses, whereas the second is for oven temperature programmed analyses. To learn more about any phases listed, or to inquire about a phase not listed, contact Technical Service at 800-359-3041 (US and Canada only), 814-359-3041, or here.
Traditional Phases: GC Column Polarity Scale

Our GC column polarity scale is a convenient tool to classify columns. The procedure we follow was proposed to us by Prof. Luigi Mondello (University of Messina, Italy). Each column is characterized with a series of five probes plus several n-alkane markers to determine the retention index for each probe. McReynolds Constants are then calculated using the retention index data of the column relative to the retention index data for the same five probes on squalane, the most non-polar GC stationary phase. The five McReynolds Constants are summed to obtain Polarity (P) values, which are then normalized to SLB®-IL100 (set at P=100) to obtain Polarity Number (P.N.) values.
Once Polarity Number (P.N.) values are calculated, the relationships to each other can be shown in a visual representation. The scale is broken into five regions. The first four regions (non-polar, intermediate polar, polar, and highly polar) are generally accepted and used by several GC column manufacturers. The fifth region (extremely polar) was required with the introduction of the SLB®-IL111 in 2010 (no column existed in this region prior to this). The positions and maximum temperatures of several of our capillary GC columns are shown (non-ionic liquid columns on the left and ionic liquid columns on the right). Our GC column polarity scale can be used for column selection because it allows multiple columns to be compared easily, because all P.N. values are relative to both squalane (0 on the scale) and SLB®-IL100 (100 on the scale).
Choose:
- Non-Polar GC columns for non-polar compounds (such as alkanes) that contain 1) only carbon and hydrogen atoms, and
2) only single bonds between carbon atoms. - Intermediate polar GC columns for an alternate selectivity of non-polar and/or polar compounds.
- Polar GC columns for polar compounds (such as alcohols, amines, carboxylic acids, diols, esters, ethers, ketones, and thiols) that contain 1) primarily carbon and hydrogen atoms, and 2) also some bromine, chlorine, fluorine, nitrogen, oxygen, phosphorus, and/or sulfur atoms.
- Highly polar GC columns for polarizable compounds (such as alkenes, alkynes, and aromatic hydrocarbons) that contain
1) only carbon and hydrogen atoms, and 2) some double and/or triple bonds between carbon atoms. - Extremely polar GC columns for additional selectivity of polarizable compounds.
Traditional Phases: Non-Polar
Non-polar GC columns are made with the least selective of the GC stationary phases. They are commonly used to separate non-polar compounds (such as alkanes) that contain 1) only carbon and hydrogen atoms, and 2) only single bonds between carbon atoms. Elution order generally follows the boiling points of the analytes.
- Interactions are primarily dispersive (van der Waals forces).
- Phases with phenyl functional groups can also undergo a moderate amount of π-π interactions.
- PTA-5 columns are specially-engineered to also allow strong basic interactions.
- Phases with octyl functional groups also possess shape selectivity.
- Application: This column, for detailed analyses of petroleum products, is known within the petroleum and chemical industries for its unique selectivity. Baseline separations of benzene/1-methylcyclopentene and toluene/2,3,3-trimethylpentane that are possible with this column are not obtainable with classical poly(dimethyl siloxane) columns.
- USP Code: None
- Phase: Bonded; poly(50% n-octyl/50% methyl siloxane)
- Temp. Limits: -60 °C to 220 °C (isothermal or programmed)
- Application: The low polarity of this column approaches squalane, making it substantially less polar than that of the widely used non-polar poly(dimethyl siloxane) columns. This column offers unique selectivity compared to non-polar and intermediate polarity columns, and can be used for confirmational analyses of PCB-containing samples.
- USP Code: None
- Phase: Bonded; poly(50% n-octyl/50% methyl siloxane)
- Temp. Limits:
–– ≤0.32 mm I.D.: -60 °C to 280 °C (isothermal or programmed)
–– ≥0.53 mm I.D.: -60 °C to 260 °C (isothermal or programmed)
- Application: These highly reproducible columns have considerable theoretical plate numbers and are designed for detailed analyses of petroleum products for PIANO, PONA, and PNA-type analytes. The 100 m version includes an extensive retention index data sheet of 400+ analytes.
- USP Code: These columns meet USP G1, G2, and G9 requirements.
- Phase: Bonded; poly(dimethyl siloxane)
- Temp. Limits: -60 °C to 320 °C (isothermal or programmed)
- Application: These columns are designed for ASTM Method D2887 (simulated distillation [Sim Dis] of petroleum fractions). Choose Petrocol® 2887 for samples having boiling points up to 1,000 °F. Use Petrocol® EX2887 for samples having boiling points greater than 1,000 °F.
- USP Code: These columns meet USP G1, G2, and G9 requirements.
- Phase: Bonded; poly(dimethyl siloxane)
- Temp. Limits:
–– Petrocol® 2887: Subambient to 350 °C (isothermal or programmed)
–– Petrocol® EX2887: Subambient to 380 °C (isothermal or programmed)
- Application: A specialized version of the SPB®-1, this column was developed for analyses of sulfur gases and other volatile sulfur compounds. The column displays relatively low column bleed, which makes it compatible for use with sulfur-specific detectors.
- USP Code: This column meets USP G1, G2, and G9 requirements.
- Phase: Bonded; poly(dimethyl siloxane)
- Temp. Limits: -60 °C to 300 °C (isothermal or programmed)
- Application: This column is designed for general purpose applications where a non-polar column is required. Analytes will be separated primarily according to boiling point.
- USP Code: This column meets USP G1, G2, and G9 requirements.
- Phase: Bonded; poly(dimethyl siloxane)
- Temp. Limits:
–– ≤0.32 mm I.D., <2 μm: -60 °C to 325 °C (isothermal) or 350 °C (programmed)
–– ≤0.32 mm I.D., ≥2 μm: -60 °C to 300 °C (isothermal or programmed)
–– ≥0.53 mm I.D., <2 μm: -60 °C to 300 °C (isothermal) or 320 °C (programmed)
–– ≥0.53 mm I.D., ≥2 μm: -60 °C to 260 °C (isothermal) or 280 °C (programmed)
- Application: This column is often used for traditional general purpose applications, where a non-polar column is required. Analytes will be separated primarily according to boiling point.
- USP Code: This column meets USP G1, G2, and G9 requirements.
- Phase: Bonded; poly(dimethyl siloxane)
- Temp. Limits:
–– ≤0.32 mm I.D., <2 μm: -60 °C to 320 °C (isothermal or programmed)
–– ≤0.32 mm I.D., ≥2 μm: -60 °C to 300 °C (isothermal or programmed)
–– ≥0.53 mm I.D., <2 μm: -60 °C to 300 °C (isothermal) or 320 °C (programmed)
–– ≥0.53 mm I.D., ≥2 μm: -60 °C to 260 °C (isothermal) or 280 °C (programmed)
- Application: The 5% phenyl equivalent phase provides a boiling point elution order with a slight increase in selectivity, especially for aromatic compounds. The low bleed characteristics, inertness, and durable nature make it the column of choice for environmental analytes (such as semivolatiles, pesticides, PCBs, and herbicides) or anywhere a low bleed non-polar column is required.
- USP Code: This column meets USP G27 and G36 requirements.
- Phase: Bonded and highly crosslinked; silphenylene polymer virtually equivalent in polarity to poly(5% diphenyl/95% dimethyl siloxane)
- Temp. Limits:
–– ≤0.32 mm I.D.: -60 °C to 340 °C (isothermal) or 360 °C (programmed)
–– ≥0.53 mm I.D.: -60 °C to 330 °C (isothermal) or 340 °C (programmed)
- Application: This rugged metal column was designed specifically for the determination of free and total glycerin in B100 biodiesel samples. A guard is integrated, thereby providing protection with a leak-free connection (the guard and analytical column are one continuous piece of tubing; there is no union between the guard and analytical column).
- USP Code: None
- Phase: Bonded; proprietary
- Temp. Limits: -60 °C to 380 °C (isothermal) or 430 °C (programmed)
- Application: This column is designed for analyses of amines and other basic analytes.
- USP Code: None
- Phase: Bonded; base-modified poly(5% diphenyl/95% dimethyl siloxane)
- Temp. Limits:
–– ≤0.32 mm I.D.: -60 °C to 320 °C (isothermal or programmed)
–– ≥0.53 mm I.D., <2 μm: -60 °C to 320 °C (isothermal or programmed)
–– ≥0.53 mm I.D., ≥2 μm: -60 °C to 260 °C (isothermal) or 280 °C (programmed)
- Application: This column is an application specific non-polar column, designed for reproducible analyses of plant sterols, cholesterol, and other animal sterols.
- USP Code: None
- Phase: Bonded; poly(5% diphenyl/95% dimethyl siloxane)
- Temp. Limits: -60 °C to 320 °C (isothermal or programmed)
- Application: This popular column is designed for general purpose applications where a non-polar column is required. The low phenyl content provides thermal stability compared to 100% poly(dimethyl siloxane) columns.
- USP Code: This column meets USP G27 and G36 requirements.
- Phase: Bonded; poly(5% diphenyl/95% dimethyl siloxane)
- Temp. Limits:
–– ≤0.32 mm I.D., <2 μm: -60 °C to 325 °C (isothermal) or 350 °C (programmed)
–– ≤0.32 mm I.D., ≥2 μm: -60 °C to 300 °C (isothermal or programmed)
–– ≥0.53 mm I.D., <2 μm: -60 °C to 300 °C (isothermal) or 320 °C (programmed)
–– ≥0.53 mm I.D., ≥2 μm: -60 °C to 260 °C (isothermal) or 280 °C (programmed)
- Application: This non-polar general purpose column provides primarily a boiling point elution order with a slight increase in selectivity, especially for aromatic compounds.
- USP Code: This column meets USP G27 and G36 requirements.
- Phase: Bonded; poly(5% diphenyl/95% dimethyl siloxane)
- Temp. Limits:
–– ≤0.32 mm I.D., <2 μm: -60 °C to 320 °C (isothermal or programmed)
–– ≤0.32 mm I.D., ≥2 μm: -60 °C to 300 °C (isothermal or programmed)
–– ≥0.53 mm I.D., <2 μm: -60 °C to 300 °C (isothermal) or 320 °C (programmed)
–– ≥0.53 mm I.D., ≥2 μm: -60 °C to 260 °C (isothermal) or 280 °C (programmed)
Traditional Phases: Intermediate Polar
Intermediate polar GC columns are made with phases that incorporate both non-polar and polar elements. Thus, they are commonly used to provide alternate selectivity to non-polar and polar columns. Elution order is determined by differences in the overall effects of possible interactions.
- Interactions are strongly dispersive (van der Waals forces). The greater the phenyl content of the phase, the stronger the interactions.
- Phases with phenyl functional groups can also undergo π-π, dipole-dipole, and dipole-induced dipole interactions. The greater the phenyl content, the stronger these interactions.
- Phases with cyanopropyl functional groups can also undergo strong dipole-dipole and moderate basic interactions. The greater the cyanopropyl content, the greater these interactions.
- Application: This column is specially tested for separation, efficiency, and low bleed. It is designed for purge-and-trap analyses of volatile halogenated, non-halogenated, and aromatic contaminants from environmental samples.
- USP Code: This column meets USP G43 requirements.
- Phase: Bonded; proprietary
- Temp. Limits:
–– ≤0.32 mm I.D.: Subambient to 250 °C (isothermal or programmed)
–– ≥0.53 mm I.D.: Subambient to 230 °C (isothermal or programmed)
- Application: This column is specially prepared and tested to meet the requirements of United States Pharmacopoeia and European Pharmacopoeia methods for determining residual solvents in pharmaceutical preparations.
- USP Code: This column meets USP G43 requirements.
- Phase: Bonded; poly(6% cyanopropylphenyl/94% dimethyl siloxane)
- Temp. Limits: -20 °C to 260 °C (isothermal or programmed)
- Application: This intermediate polarity column, designed for analyses of volatile organic compounds (VOCs), offers great retention and resolution of highly volatile compounds. Use this column in direct injection ports or coupled to purge-and-trap systems.
- USP Code: None
- Phase: Bonded; proprietary
- Temp. Limits:
–– ≤0.32 mm I.D., <2 μm: Subambient to 250 °C (isothermal or programmed)
–– ≤0.32 mm I.D., ≥2 μm: Subambient to 230 °C (isothermal or programmed)
–– ≥0.53 mm I.D., <2 μm: Subambient to 250 °C (isothermal or programmed)
–– ≥0.53 mm I.D., ≥2 μm: Subambient to 230 °C (isothermal or programmed)
- Application: This column has intermediate polarity due to the higher (20%) phenyl content, producing a different elution order of polar compounds for confirmational information. It is often used for analyses of aromatic analytes.
- USP Code: This column meets USP G32 requirements.
- Phase: Bonded; poly(20% diphenyl/80% dimethyl siloxane)
- Temp. Limits: -25 °C to 300 °C (isothermal or programmed)
- Application: Increased phase polarity, due to cyanopropylphenyl functional group substitution, offers unique selectivity compared to other phases. This column works well with systems employing ECD, NPD, and MSD detectors, and is often used for alcohols, oxygenates, pharmaceuticals, pesticides, and PCB applications.
- USP Code: This column meets G46 requirements
- Phase: Bonded; poly(14% cyanopropylphenyl/86% dimethyl siloxane)
- Temp. Limits:
–– ≤0.32 mm I.D.: Subambient to 280 °C (isothermal or programmed)
–– ≥0.53 mm I.D.: Subambient to 260 °C (isothermal or programmed)
- Application: This column is specially tested with low concentrations of 18 chlorinated pesticides, using an ECD detector. In addition to selectivity and efficiency, it is also tested to ensure minimum breakdown of 4,4’-DDT and endrin. This column is also suitable for use in herbicide analyses.
- USP Code: None
- Phase: Bonded; proprietary
- Temp. Limits: Subambient to 300 °C (isothermal or programmed)
- Application: With a phenyl content of 35%, this column offers a higher polarity option compared to columns containing a lower phenyl content. This column is useful for analyses of polar compounds because they are retained longer relative to non-polar compounds.
- USP Code: This column meets USP G42 requirements.
- Phase: Bonded; poly(35% diphenyl/65% dimethyl siloxane)
- Temp. Limits: 0 °C to 300 °C (isothermal or programmed)
- Application: This column has the highest phenyl content of the common phenyl-containing series of phases. The column is useful for analyses of polar analytes and provides useful confirmational information. It also offers additional selectivity for polynuclear aromatic hydrocarbon isomers over columns with lower phenyl content.
- USP Code: This column meets USP G3 requirements.
- Phase: Bonded; poly(50% diphenyl/50% dimethyl siloxane)
- Temp. Limits: 30 °C to 310 °C (isothermal or programmed)
Traditional Phases: Polar
Polar GC columns are made using polar stationary phases, the most common being polyethylene glycol and modified versions. These columns are commonly used to separate polar analytes (such as alcohols, amines, carboxylic acids, diols, esters, ethers, ketones, and thiols) that contain 1) primarily carbon and hydrogen atoms, and 2) also some bromine, chlorine, fluorine, nitrogen, oxygen, phosphorus, and/or sulfur atoms. Elution order is determined by differences in the overall effects of possible interactions.
- Dispersive (van der Waals forces), π-π, dipole-dipole, and dipole-induced dipole interactions are all strong with these columns.
- Moderate amounts of hydrogen bonding and basic interactions are also possible.
- SPB®-1000 and NUKOL™ columns are specially-engineered to also allow strong acidic interactions.
- Carbowax® amine columns are specially-engineered to also allow strong basic interactions.
- Application: Supelco offers the broadest range of cyanopropyl columns in the industry, such as this intermediate polarity column.
- USP Code:This column meets USP G7 and G19 requirements.
- Phase: Bonded; poly(50% cyanopropylphenyl/50% dimethyl siloxane)
- Temp. Limits: 45 °C to 220 °C (isothermal) or 240 °C (programmed)
- Application: This column provides the necessary polarity for analyses of polyunsaturated fatty acids (PUFAs) as fatty acid methyl esters (FAMEs). This column is specifically tuned to provide highly reproducible analyses.
- USP Code:This column meets USP G18 requirements.
- Phase: Bonded; poly(alkylene glycol)
- Temp. Limits: 50 °C to 220 °C (isothermal or programmed)
- Application: The incorporation of acid functional groups into the phase lends an acidic character to this column, useful for analyses of volatile acidic compounds. It offers great performance for analyses of glycols. It is the recommended column for ethylene glycol analysis.
- USP Code:This column meets USP G25 and G35 requirements.
- Phase: Bonded; acid-modified poly(ethylene glycol)
- Temp. Limits: 60 °C to 200 °C (isothermal) or 220 °C (programmed)
- Application: The incorporation of acid functional groups into the phase lends an acidic character to this column, useful for analyses of volatile acidic compounds. Difficult to analyze carboxylic acids (free fatty acids) can be analyzed with excellent peak shape and minimal adsorption.
- USP Code:This column meets USP G25 and G35 requirements.
- Phase: Bonded; acid-modified poly(ethylene glycol)
- Temp. Limits: 60 °C to 200 °C (isothermal) or 220 °C (programmed)
- Application: This specially prepared base-deactivated column is designed for analyses of primary, secondary, and tertiary amines, as well as other volatile basic compounds.
- USP Code:None.
- Phase: Non-bonded; base-modified poly(ethylene glycol)
- Temp. Limits: 60 °C to 200 °C (isothermal or programmed)
- Application: This column allows highly reproducible analyses of fatty acid methyl esters (FAMEs), specifically the omega 3 and omega 6 fatty acids. It is tested to ensure reproducible FAME equivalent chain length (ECL) values and resolution of key components.
- USP Code:This column meets USP G16 requirements.
- Phase: Bonded; poly(ethylene glycol)
- Temp. Limits: 50 °C to 280 °C (isothermal or programmed)
- Application: This column is based on one of the most widely used polar phases, Carbowax™ 20M, and is a polar column suitable for analyses of solvents, fatty acid methyl esters (FAMEs), food, flavor and fragrance compounds, alcohols, and aromatics. Additionally, this column is a great choice when a polar general purpose column is required.
- USP Code:This column meets USP G16 requirements.
- Phase: Bonded; poly(ethylene glycol)
- Temp. Limits:
–– ≤0.32 mm I.D.: 35 °C to 280 °C (isothermal or programmed)
–– ≥0.53 mm I.D., <2 μm: 35 °C to 280 °C (isothermal or programmed)
–– ≥0.53 mm I.D., ≥2 μm: 35 °C to 250 °C (isothermal or programmed)
- Application: Selectivity more polar than PEG/wax phases, resulting in unique elution patterns. Higher maximum temperature than PEG/ wax columns (300 °C compared to 270–280 °C). Great choice for analysis of neutral and moderately basic analytes.
- USP Code:None
- Phase: Non-bonded; 1,12-di(tripropylphosphonium)dodecane bis(trifluoromethylsulfonyl)imide
- Temp. Limits: Subambient to 300 °C (isothermal or programmed)
- Application: Modified (deactivated) version of SLB®-IL59 provides better inertness. Selectivity more polar than PEG/wax phases, resulting in unique elution patterns. Higher maximum temperature than PEG/wax columns (300 °C compared to 270–280 °C). Excellent alternative to existing PEG/wax columns. Also a good GCxGC column choice.
- USP Code:None
- Phase: Non-bonded; 1,12-di(tripropylphosphonium)dodecane bis(trifluoromethylsulfonyl)imide
- Temp. Limits: 35 °C to 300 °C (isothermal or programmed)
- Application: The first of our third generation ionic liquid columns. Modified (triflate anion) version of SLB®-IL59 increases inertness. Selectivity more polar than PEG/wax phases, resulting in unique elution patterns. Higher maximum temperature than PEG/wax columns (290 °C compared to 270–280 °C). Great choice for analysis of neutral and moderately basic analytes.
- USP Code:None
- Phase: Non-bonded; 1,12-di(tripropylphosphonium)dodecane bis(trifluoromethylsulfonyl)imide trifluoromethylsulfonate
- Temp. Limits: 40 °C to 290 °C (isothermal or programmed)
Traditional Phases: Highly Polar
Highly polar GC columns are made with very selective GC stationary phases, typically containing high percentages of cyanopropyl functional groups. They are commonly used to analyze polarizable compounds (such as alkenes, alkynes, and aromatic hydrocarbons) that contain 1) only carbon and hydrogen atoms, and 2) some double and/or triple bonds between carbon atoms. Elution order is determined by differences in the overall effects of possible interactions.
- Strong dispersive (van der Waals forces), very strong dipole-dipole, very strong dipole-induced dipole, and moderate basic interactions are possible. The greater the cyanopropyl content of the phase, the greater these interactions.
- Application: Supelco offers the broadest range of biscyanopropyl phases in the industry. This column is a highly specialized column that offers both polar and polarizable features due to the substitution of biscyanopropyl and phenyl groups onto the polymer backbone. It can be used for both high and low temperature separations for analytes such as geometric isomers of fatty acid methyl esters (FAMEs), dioxins, and aromatic compounds.
- USP Code: This column meets USP G8 requirements.
- Phase: Non-bonded; poly(80% biscyanopropyl/20% cyanopropylphenyl siloxane)
- Temp. Limits: Subambient to 250 °C (isothermal or programmed)
- Application: The first of our second generation ionic liquid columns. Phase structure engineered with numerous interaction mechanisms, resulting in selectivity differences even when compared to columns with similar GC column polarity scale values.
- USP Code: None
- Phase: Non-bonded; tri(tripropylphosphoniumhexanamido)triethylamine bis(trifluoromethylsulfonyl)imide
- Temp. Limits: Subambient to 270 °C (isothermal or programmed)
- Application: A highly polar cyanosiloxane column specially tested for analyses of dioxins, specifically tetrachlorodibenzodioxin (TCDD) isomers. Because the phase is stabilized, it has a maximum temperature slightly higher than non-bonded cyanosiloxane columns.
- USP Code: None
- Phase: Stabilized; proprietary
- Temp. Limits: Subambient to 275 °C (isothermal or programmed)
- Application: A highly polar cyanosiloxane column commonly used for separation of geometric (cis/trans) fatty acid methyl ester (FAME) isomers as a group. Also useful when a highly polar general purpose column with good thermal stability is required.
- USP Code: This column meets USP G48 requirements.
- Phase: Stabilized; poly(90% biscyanopropyl/10% cyanopropylphenyl siloxane)
- Temp. Limits: Subambient to 275 °C (isothermal or programmed)
- Application: This highly polar biscyanopropyl column was specifically designed for detailed separation of geometric-positional (cis/trans) isomers of fatty acid methyl esters (FAMEs). It is extremely effective for FAME isomer applications.
- USP Code: This column meets USP G5 requirements.
- Phase: Non-bonded; poly(biscyanopropyl siloxane)
- Temp. Limits: Subambient to 250 °C (isothermal or programmed)
- Application: This non-bonded column offers the highest polarity in its class. As with all general purpose biscyanopropyl columns, it is highly effective for both high and low temperature separations of geometric isomers of fatty acid methyl esters (FAMEs), dioxins, carbohydrates, and aromatic compounds.
- USP Code: This column meets USP G5 requirements.
- Phase: Non-bonded; poly(biscyanopropyl siloxane)
- Temp. Limits: Subambient to 250 °C (isothermal or programmed)
- Application: Selectivity slightly more polar than polysiloxane phases with a high percentage of cyanopropyl pendent groups, resulting in unique elution patterns. Great choice for analysis of neutral and moderately basic analytes.
- USP Code: None
- Phase: Non-bonded; 1,12-di(2,3-dimethylimidazolium)dodecane bis(trifluoromethylsulfonyl)imide
- Temp. Limits: 50 °C to 270 °C (isothermal or programmed)
- Application: The unique chemistry of the phase allows for specialized separations. It is often used for analyses of alcohols and aromatics in mineral spirits, aliphatic constituents in gasoline, impurities in individual aromatics, and oxygenates.
- USP Code: None
- Phase: Non-bonded; 1,2,3-tris(2-cyanoethoxy)propane
- Temp. Limits: Subambient to 145 °C (isothermal or programmed)
- Application: World’s first commercially available ionic liquid GC column. Serves as the benchmark of 100 on our GC column polarity scale. Selectivity almost identical to TCEP phase. Higher maximum temperature than TCEP columns (230 °C compared to 140 °C). Great choice for analysis of neutral and polarizable (contain double and/or triple C-C bonds) analytes.
- USP Code: None
- Phase: Non-bonded; 1,9-di(3-vinylimidazolium)nonane bis(trifluoromethylsulfonyl)imide
- Temp. Limits: Subambient to 230 °C (isothermal or programmed)
Traditional Phases: Extremely Polar
Extremely polar GC columns are made with the most selective of the GC stationary phases. They are commonly used to provide alternative selectivity of polarizable compounds. Another use is in GCxGC applications due to their orthogonal selectivity to non-polar columns. Elution order is determined by differences in the overall effects of possible interactions.
- Strong dispersive (van der Waals forces), very strong dipole-dipole, very strong dipole-induced dipole, and moderate basic interactions are possible.
- Application: World’s first commercial column to rate over 100 on our GC column polarity scale. Selectivity most orthogonal to non-polar and intermediate polar phases, resulting in very unique elution patterns. Maximum temperature of 270 °C is very impressive for such an extremely polar column. Great choice for separation of polarizable analytes (contain double and/or triple C-C bonds) from neutral analytes. Also a good GCxGC column choice.
- USP Code: None
- Phase: Non-bonded; 1,5-di(2,3-dimethylimidazolium)pentane bis(trifluoromethylsulfonyl)imide
- Temp. Limits: 50 °C to 270 °C (isothermal or programmed)
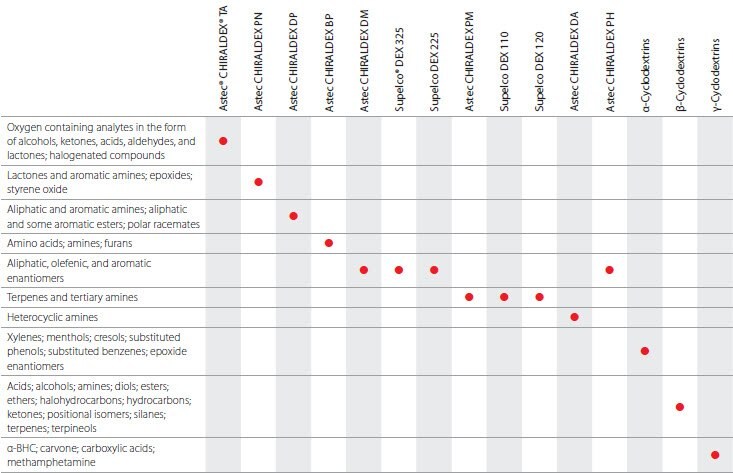
Chiral Phases
Chiral GC phases consist of derivatives of α-, β-, or γ-cyclodextrin for the separation of enantiomers. These phases can routinely separate a variety of underivatized non-aromatic enantiomers and several aromatic enantiomers that remain difficult to resolve by HPLC. These phases specifically and effectively separate many of these types of molecules, including thousands of compounds that are starting materials or intermediates for chiral synthesis, biochemical and pharmaceutical intermediates and metabolites, environmental contaminants, flavors, etc. The brochure “Astec® CHIRALDEX® and Supelco® DEX™ Chiral GC Columns: The Widest Variety of Derivatized Cyclodextrins” (T411101, OEM) contains valuable information concerning chiral GC columns, and includes selection guidelines. A copy of this brochure can be obtained at no-charge by contacting Technical Service at 800-359-3041 (US and Canada only), 814-359-3041, or here.
- Application: These columns are used for analyses of enantiomers to determine biological activity (pharmaceutical industry), aroma (flavor and fragrance and food and beverage industries), whether hazardous (environmental industry), and purity (chemical industry).
- USP Code: None
- Phase: Fourteen specialized phase chemistries comprised of complex derivatives of cyclodextrins that impart a broad range of selectivities
- Temp. Limits:
–– TA Phases: -10 °C to 180 °C (isothermal or programmed)
–– All Other Phases: -10 °C to 200 °C (isothermal) or 220 °C (programmed)
- Application: These columns are used for analyses of enantiomers to determine biological activity (pharmaceutical industry), aroma (flavor and fragrance and food and beverage industries), whether hazardous (environmental industry), and purity (chemical industry).
- USP Code: None
- Phase: Ten unique phases comprised of derivatives of cyclodextrins that are able to perform many enantiomeric separations
- Temp. Limits: 30 °C to 230 °C (isothermal or programmed)
PLOT Columns
We offer a wide variety of Porous Layer Open Tubular (PLOT) GC columns, including those made with our specialty carbon adsorbents. A proprietary procedure is used to fix adsorbent particles to the inside of fused silica tubing, and ensures they will not be dislodged in normal use. PLOT GC columns are commonly used for separations of small molecules, such as permanent gases, light hydrocarbons, and volatile sulfur compounds. Choose:
- Carboxen®-1010 PLOT for separations of hydrogen, oxygen, nitrogen, carbon monoxide, methane, carbon dioxide, and C2/C3 hydrocarbons. This is the only column that can separate all these permanent gases.
- Carboxen-1006 PLOT for most permanent gases and C1-C3, using above ambient initial temperatures. Also for resolving formaldehyde/water/methanol (formalin) mixtures and monitoring impurities in ethylene.
- Supel-Q™ PLOT for analyses of sulfur gases, alcohols, ketones, aldehydes, and many polar compounds. Also for carbon dioxide and C1-C4 hydrocarbons at above ambient temperatures, and for gasoline and other petroleum fractions.
- Alumina sulfate PLOT for C1-C4 hydrocarbons, specifically methane from the C2 hydrocarbons, with reduced peak tailing. Also for elution of acetylene after n-butane, and the elution of methyl acetylene after n-pentane and 1,3-butadiene.
- Alumina chloride PLOT for C1-C4 hydrocarbons. Also for excellent separation of many common fluorocarbon compounds
- Mol Sieve 5A PLOT for oxygen, nitrogen, carbon monoxide, and methane in less than 5 minutes. For more difficult separations, such as argon from oxygen, by using subambient temperatures (15 °C or below).
- Application: This column is ideal for the separation of all major components in permanent gas (helium, hydrogen, oxygen, nitrogen, carbon monoxide, methane, and carbon dioxide) and light hydrocarbons (C2-C3) in the same analysis. It is the only column commercially available that is able to separate all major components in permanent gas. This column can also separate oxygen from nitrogen at subambient temperatures.
- USP Code: None
- Phase: Carbon molecular sieve
- Temp. Limits: Subambient to 250 °C (isothermal or programmed)
- Application: This column is ideal for the separation of many permanent gas components (such as helium, hydrogen, nitrogen, carbon monoxide, methane, and carbon dioxide), and light hydrocarbons (C2-C3) in the same analysis. It is ideal for resolving formaldehyde/water/methanol (formalin) mixtures and monitoring impurities in ethylene. This column can be used with high flow rates and rapid temperature programs to ensure excellent, fast separations.
- USP Code: None
- Phase: Carbon molecular sieve
- Temp. Limits: Subambient to 250 °C (isothermal or programmed)
- Application: This column exhibits very little bleed, even at its maximum temperature, and effectively resolves carbon dioxide and C1-C4 hydrocarbons at above ambient temperatures. It is also suitable for analyses of sulfur gases, alcohols, ketones, aldehydes, and many polar compounds. Gasoline and other petroleum fractions can be analyzed as well.
- USP Code: None
- Phase: Divinylbenzene
- Temp. Limits: Subambient to 250 °C (isothermal or programmed)
- Application: This highly dependable column has the necessary selectivity for the separation of alkanes, alkenes, and alkynes in mixtures of C1-C4 hydrocarbons. It provides elution of acetylene after n-butane and the elution of methyl acetylene after n-pentane and 1,3-butadiene. The polymer surface is deactivated to reduce peak tailing.
- USP Code: None
- Phase: Sulfate-deactivated alumina
- Temp. Limits: Subambient to 180 °C (isothermal or programmed)
- Application: This column allows for the separation of C1-C4 hydrocarbons. Because this column is slightly less polar than the Alumina sulfate PLOT, it provides a different elution order pattern when alkane, alkene, and alkyne mixtures of light hydrocarbons are analyzed. It also provides excellent separation of many common fluorinated compounds, such as freons.
- USP Code: None
- Phase: Chloride-deactivated alumina
- Temp. Limits: Subambient to 180 °C (isothermal or programmed)
- Application: This column can be used for the separation of many permanent gas components, such as oxygen, nitrogen, carbon monoxide, and methane, in less than five minutes. More difficult separations, such as argon from oxygen, can be achieved by using subambient temperatures. These columns possess the strongest adsorption strength of any PLOT column.
- USP Code: None
- Phase: Aluminosilicate
- Temp. Limits: Subambient to 300 °C (isothermal or programmed)
SCOT Columns
Supelco is the leader in Support Coated Open Tubular (SCOT) GC column technology. Our unsurpassed manufacturing technique allows us to deposit a uniform layer of liquid phase-coated support particles on the inner wall of stainless steel tubing. This technology gives us access to many phases that are inaccessible to conventional fused silica capillary column manufacturing technology. SCOT columns combine the sensitivity and excellent sample resolution of capillary GC with the extensive stationary phase library of packed GC.
All our SCOT columns have dimensions of 50 feet x 1/32 inch O.D. x 0.02 inch I.D. and include 1/16 inch O.D. connections at each end. They are banded in 3.5 inch coils, with 12 inch loose column at each end. Four columns are available as stock items. Columns with other phases may be available through our custom program.
- Application: Use for analyses of xylene isomers.
- USP Code: None
- Phase: Bentone 34/di-n-decyl phthalate
- Temp. Limits: 10 °C to 150 °C (isothermal or programmed)
- Application: Use for analyses of aromatic analytes.
- USP Code: None
- Phase: 1,2,3-Tris(2-cyanoethoxy)propane
- Temp. Limits: 0 °C to 150 °C (isothermal or programmed)
- Application: Use for analyses of olefins.
- USP Code: None
- Phase: Bis-methoxyethyladipate
- Temp. Limits: 25 °C to 100 °C (isothermal or programmed)

*Plus an integrated 2 m x 0.53 mm I.D. guard.
**Wound onto a 5 inch cage to fit an Agilent 6850 GC.
To continue reading please sign in or create an account.
Don't Have An Account?