The Utility of Headspace Grade Solvents for the Analysis of Organic Volatile Impurities
Section Overviews
Introduction
Organic volatile impurities or “OVIs” sometimes remain behind in pharmaceutical preparations as a result of synthetic and manufacturing procedures. For reasons of health and safety, testing is done to ensure that these solvents are not above concentration limits listed by the United States Pharmacopeia (USP) and in the International Conference on Harmonization (ICH) guidelines (1,2). Static headspace GC (SH-GC) is a commonly used technique in the analysis of these OVIs. This technique concentrates volatile analytes, and allows for their analysis free from sample matrix.
Samples to be analyzed by SH-GC must be dissolved in a suitable solvent; and in addition to being able to dissolve the sample, the solvent chosen must allow for sufficient sensitivity of the analytes of interest in the headspace. Partition coefficient affect the ability of an analyte to enter into the headspace from the liquid sample, and those with lower values will have higher vapor pressure and thus greater sensitivity in the headspace (3). Thus, for low-level detection, the analytes of interest must have low partition coefficients in the dissolution solvent chosen. In addition, the vapor pressure of the dissolution solvent itself should be sufficiently low so it will not affect detection of OVI analytes by “flooding” the headspace.
Water offers a very low partition coefficient for analytes and has a low vapor pressure, but cannot be used in all cases. USP <467> and European Pharmacopeia (EP) methodologies list procedures for both water-soluble and water-insoluble samples. For water-insoluble samples, USP <467> designates the use of the solvents dimethyl sulfoxide (DMSO) and dimethylformamide (DMF). Other dissolution solvents that have been found to be useful for headspace analysis of water-insoluble samples include dimethylacetamide (DMAC) and 1,3-dimethyl-2-imidazolidinone (DMI), and the later is described for use in EP Method 2.4.24 (4). These solvents elute later than most OVI analytes in chromatographic analyses and have significantly lower vapor pressure than many other high boiling-point organic compounds.
The purity of dissolution solvents is essential to avoid extraneous peaks in the chromatographic analysis and prevent interference with the analytes of interest. Many protocols followed by laboratories doing OVI analysis require the analysis of an acceptable blank, and some published methodologies, such as EP Method 2.4.24, require the analysis of a blank to verify the absence of interfering peaks. High purity headspace grade solvents are now available which are specially tested to ensure suitability for SH-GC analysis.
Experimental
In this evaluation, the utility of DMSO headspace grade solvent was evaluated for use in the SH-GC analysis of OVIs. The purity of the headspace solvent was evaluated by preparing sample blanks using headspace and alternative grades of DMSO. The retention times of peaks present in the blanks were then compared to an OVI standard prepared in headspace grade DMSO solvent.
The blanks were prepared by pipetting 1 mL of DMSO into a 10 mL headspace vial, and subjecting the sealed vial to SH-GC analysis. The OVI working standard included a variety of common process solvents, representing various classes as described in USP <467> and ICH guidelines. It was prepared from a stock solution in DMAC by dilution with headspace grade DMSO. The composition and final concentration of the OVI working standard is summarized in Table 1. The working standard was prepared for SH-GC analysis in a similar manner to the blanks. Both blanks and the standard were analyzed by SH-GC using the parameters listed in Table 2.
Results
Chromatograms of the SH-GC blanks using headspace and organic synthesis grades of DMSO are presented in Figures 1 and 2. Overall, the headspace grade blank had fewer peaks in the OVI elution range than the organic synthesis grade blank. Comparing the blanks with the chromatogram of the working OVI standard prepared in headspace-grade DMSO (Figure 3), both blanks were found to contain some DMF. The organic synthesis blank contained a peak eluting close to the retention time of ethanol. This peak could potentially interfere with the proper detection and analysis of ethanol as an OVI. A peak corresponding to the retention time of DMI was detected in the SH-GC blank. This same peak was also detected in the OVI working standard, which was prepared in headspace grade DMSO.
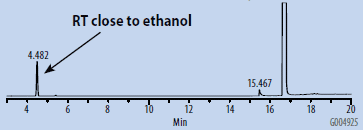
Figure 1. SH-GC Blank, DMSO – Organic Synthesis Grade
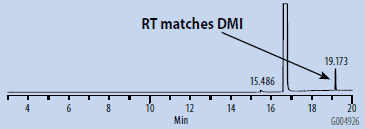
Figure 2. SH-GC Blank, DMSO – Headspace Grade
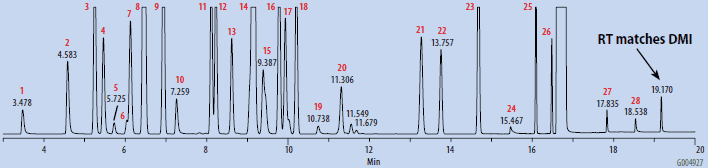
Figure 3. SH-GC Analysis of OVI Working Standard Prepared in Headspace Grade DMSO
Conclusion
The headspace grade produced a “cleaner” blank for SH-GC analysis than a lesser grade of DMSO. The peaks that did appear in the headspace grade solvent blank eluted outside of the retention range of the OVIs analyzed. Additional headspace grade solvents (DMF, DMAC, and DMI) suitable for this application are also available. Experimental data on these solvents, similar to that presented here for DMSO, can be obtained by requesting Supelco Publication T409180 (only available electronically).
Acknowledgments
We would like to thank Amanda Quiroga and Michael Dong at Genentech USA, for providing the data for this evaluation, and for their invaluable input.
References
To continue reading please sign in or create an account.
Don't Have An Account?