Universal HPTLC Mix (UHM) for Simplified System Suitability Tests: A Novel Concept for HPTLC Suitability Test
Tiên Do, Head of Laboratory1, Eike Reich, Chief Scientific Officer1, Hanspeter Sprecher, R&D Scientist2, Matthias Nold, Product Manager Reference Materials2
1CAMAG, 2MilliporeSigma
Abstract
We recently launched the HPTLC calibration mix (cat. no. 91816) for use as a universal system suitability test (SST) solution, developed in collaboration with CAMAG, a leading manufacturer of HPTLC instrumentation.
Introduction
In HPTLC, the SST often qualifies only a limited region of the chromatogram (e.g., specific RF values or narrow RF ranges). If no deviation from the acceptance criteria is observed, the entire chromatographic system is typically considered compliant. However, in practice, the chromatographic quality of the other regions remains unknown. Additionally, HPTLC methods using developing solvents of different polarities resulting in different selectivity’s may require different sets of substances for different SST. Cost and stability are the other criteria to consider when selecting reference substances for a system suitability test. To offer convenience and reliability, a Universal HPTLC Mix (UHM) for use in SST was developed, that is applicable for use with a wide variety of solvents.1
The idea for a universal system suitability test (SST) for HPTLC originated from the company Anchrom (India). Dr. Manjusha Phanse started the evaluation of this concept. Thinking about the practical aspects of qualifying an HPTLC analysis and the needs of clients for routine analysis, the laboratory teams of CAMAG and Anchrom worked together to create a new SST concept for HPTLC. This project was later supported by Sigma-Aldrich Chemie GmbH (subsidiary of Merck KGaA, Darmstadt, Germany). The outcome was a joint publication in the Journal of Chromatography A1 and the launch of the HPTLC calibration mix, a ready-to-use analytical standard solution, suitable for the CAMAG SST concept.
This mix is applicable for SST in a wide range of chromatographic systems, with different polarities and selectivities. The replacement of conventional substances for SST by the UHM will help laboratories to save time and money required for laborious in-house investigations of specific reference substances for each method to be qualified. Different fields of application can benefit from the UHM concept, such as herbal drugs, forensics, pharmaceuticals, cosmetics, etc.
Definition of Mix Composition
In the first step of the investigation, suitable substances for the UHM were selected. An initial list of 56 candidates was determined using the following criteria:
- low hazard (not harmful and non-toxic substances)
- detectability at UV 254 and 366 nm prior to derivatization
- high stability in solution
The chromatographic behavior of those 56 compounds was evaluated with 20 developing solvents (8 are shown in Table 1), covering a wide range of polarities and selectivities.
Chromatographic Conditions
Plate: HPTLC plates silica gel 60 F254, 20×10 cm (1.05642).
Standard solutions: In the development phase, 2.0 µL of individual compound solutions were applied as bands with the Automatic TLC Sampler (ATS 4), band length 8.0 mm, distance from left edge 20.0 mm, distance from lower edge 8.0 mm. For the HPTLC calibration mix, an application volume of 2.0 µL is recommended for best results.
Chromatography: Plates were developed to 70 mm (from the lower edge) in the ADC 2 with chamber saturation (20 min, with saturation pad) and after activation at 33% relative humidity for 10 min using a saturated aqueous solution of magnesium chloride. 20 different developing solvents (eight of them are listed in Table 1) were investigated, followed by drying for 5 min.
Documentation: Images of the plates were captured with the TLC Visualizer 2 at UV 254 nm and 366 nm.
Densitometry: Absorbance measurement at 254 nm and fluorescence measurement at 366 nm with TLC Scanner 4 and visionCATS, slit dimension 5.00 mm x 0.20 mm, scanning speed 20 mm/s. For the fluorescence measurement, a mercury lamp and a cut- off filter 400 nm were used.
The objective was to find the ideal set of substances that provides an even distribution of zones throughout the entire chromatogram for a maximum number of different developing solvents. Additionally, each developing solvent should achieve a baseline separation for at least 3–4 substances. The finally chosen substances and their chromatograms with eight different developing solvents are shown in Figure 1.

Figure 1.Substances selected for UHM and the HPTLC chromatograms of the UHM with eight different developing solvents (Table 1). Bands: 1. Guanosine, 2. Sulisobenzone, 3. Thymidine, 4. Paracetamol, 5. Phthalimide, 6. 9-Fluorenol (9-Hydroxyfluorene), 7. Thioxanthone, 8. Octrizole (2-(2H-Benzotriazol-2-yl)-4-(1,1,3,3-tetramethylbutyl)phenol).
To evaluate, whether the proposed UHM responds to variations in the chromatographic conditions, three experiments were performed:
In the first, plates were conditioned to different relative humidities (from 0% to 90%) prior to development. As shown in Figure 2, the UHM is sensitive to variations in relative humidity, particularly to the higher ones. The differences were more pronounced for developing solvents containing no water.
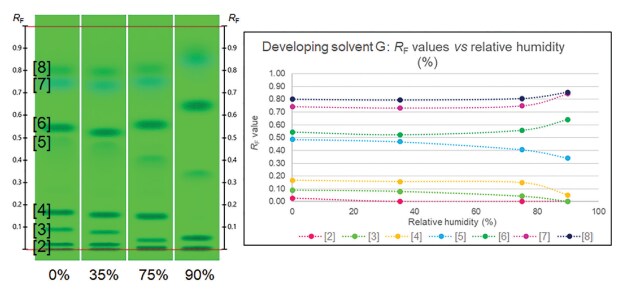
Figure 2.UHM evaluated with developing solvent G (Table 1) and conditioned with different relative humidities prior to development. (Bands: 1. Guanosine, 2. Sulisobenzone, 3. Thymidine, 4. Paracetamol, 5. Phthalimide, 6. 9-Fluorenol, 7. Thioxanthone, 8. Octrizole).
In the second experiment, the individual proportion of the solvents in developing solvents B and F (Table 1) was changed (±10%), and the effect on the chromatography was evaluated. A difference of up to 0.06 RF units could be observed from the mean RF values of the control track.
In the third experiment, different levels of chamber saturation were tested: unsaturated, partially saturated (20 min, no saturation pad), and saturated (20 min, with saturation pad). RF values increased with partial saturation, but then decreased with full saturation (Figure 3), proving that the SST with the UHM may indicate chamber saturation problems.
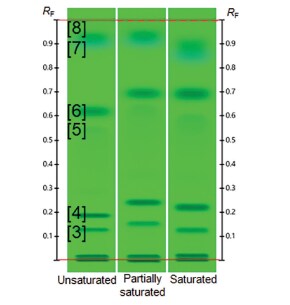
Figure 3.UHM evaluated with developing solvent G (Table 1) developed with different levels of chamber saturation (Bands: 1. Guanosine, 2. Sulisobenzone, 3. Thymidine, 4. Paracetamol, 5. Phthalimide, 6. 9-Fluorenol, 7. Thioxanthone, 8. Octrizole).
The UHM performance was evaluated in intra- and inter-laboratory tests based on the ΔRF in developing solvents B, F and G. For the intra-laboratory test, the confidence interval ΔRF was 0.03, while for the inter-laboratory test, this value was 0.04.
Throughout the development of the final composition, we supported CAMAG with the individual components that were considered and at a later stage with several prototypes of the mix. The subsequent optimization lead to the final composition (Table 2).
The ready-to-use standard mix is available as cat. no. 91816. This product is manufactured under ISO 9001 management system as an analytical standard quality grade and is provided in a 1 mL amber glass ampoule. Stability checks were preformed to ensure that the mix is fit for purpose for the entire duration of the shelf life.
Conclusion
The newly developed universal HPTLC mix (UHM) enables HPTLC users to efficiently and reliably perform their system suitability testing (SST).
References
To continue reading please sign in or create an account.
Don't Have An Account?