Water for pH Measurement and Buffer Preparation
Section Overview
- Why is pH Measured?
- Impact of Water Quality on pH Measurements and Buffer Preparation
- What is the pH of Ultrapure Water?
- Experiment: Impact of Buffer Dilution on pH Measurements
- Lab Water Solutions for Measuring pH and Preparing Buffers
- Related Products
Why is pH Measured?
The measurement of pH is one of the most widely performed tests in laboratories and industries. This is because many physical properties and chemical and biological processes are dependent on the acidity and basicity of solutions, which are measured by pH. The following are all influenced by pH:
- Solubility of many chemicals
- Bioavailability of drug molecules
- Rates of chemical reactions
- Physiological chemistry of living organisms
pH measurement is so common that almost everything we use has been tested for pH at one point: our shampoos and soaps, our tap water, everything we eat and drink, and our medicines, to name a few.
How is pH Measured?
A pH meter is the most accurate way to measure pH. The pH measurement is based on the difference of potential between two electrodes, one of them being a reference electrode. An approximate measure of pH may be obtained by using a pH indicator, a substance that changes color around a particular pH value. Universal indicators may also be used. They consist of a mixture of indicators such that there is a continuous color change from about pH 2 to pH 10. The pH value is measured semiquantitatively by colorimetric determination. ‘pH paper’ or ‘pH test strips’ or “pH-indicator strips” are cellulose strips with covalently bound special indicator dyes.
Impact of Water Quality on pH Measurements and Buffer Preparation
A buffer is a solution that maintains its pH after the addition of a small amount of an acid or a base. Buffers are widely used in laboratories since pH influences the rate of many chemical and enzymatic reactions.
Buffers and solutions used to measure pH are prepared from solid reagents dissolved in water, or from pre-made concentrated solutions that can be diluted with water. The water used to prepare these solutions should be pure, devoid of significant concentrations of acids or bases, and free of substances that can modify the pH of the solutions.
Impact of Water Contaminants on pH
Ions: Significant contamination by ions, such as ammonium and carbonate, can alter the hydrogen ion concentration of a solution, resulting in inaccurate pH measurements. When preparing buffers of specific ionic strength, the presence of all ions at high concentration will modify the ionic strength of the solutions. It is therefore best to select water with low ionic concentration; a resistivity > 1 MΩ.cm is recommended.
Organics: Water that is heavily contaminated with weak organic acids or bases could modify the pH of a solution. To ensure low concentration of these organic molecules, the overall concentration of organics in the water should be low. Water with a Total Organic Carbon (TOC) below 50 ppb should be selected.
Bacteria: While bacteria would not withstand strong acids or bases, they may develop in buffers at pH between 5 and 9. In addition, bacteria release charged organics when their membrane is disrupted. Water with low bacterial content would avoid any problem linked to bacteria and their by-products.
Particles: Although not very sensitive to particles, the performance of a pH meter could be affected by high concentration of particles, which could aggregate on the surface of the electrodes. A filtration step at the end of the water purification process is recommended.
How Storing Water May Impact pH Measurements
Water storage can have a dramatic effect on the final pH of solutions, in particular when pure water is stored before preparing the solutions. After purification, water is very pure and ions and gases can dissolve readily. Carbon dioxide from the air can dissolve in water to generate bicarbonate and carbonate, essentially turning the stored pure water into a carbonate buffer. Mixing that carbonate solution with pre-made buffers or with powders is likely to result in a pH different than the expected.
In addition, bacteria can develop readily in stored water, and they will create biofilms in the container used to store the water and solutions/buffers. It is therefore highly recommended to clean thoroughly the bottles used to prepare and keep the buffers between each use.
What is the pH of Ultrapure Water?
The theoretical pH of ultrapure water is 6.998 (Figure 1).1-2 However, one cannot measure the pH of ultrapure water simply by dipping the electrodes of a pH meter into water. As ultrapure water is very pure, it contains very few ions and has a low buffering capacity. Its pH value can therefore change quickly once it has been dispensed from the system. Any impurity on the surface of the container used to collect ultrapure water will dissolve and change the pH value. Additionally, carbon dioxide naturally present in the air will dissolve in the water, producing bicarbonate and decreasing the pH value to approximately 5.8.
pH meters are not designed to operate in a solution containing almost no ions; they are not adapted to measure the pH of ultrapure water and may therefore deliver inaccurate results. Some companies have designed pH meters able to measure the pH of ultrapure water in dynamic operating conditions, but these are rarely used in regular research laboratories.
For these reasons, it is recommended to use resistivity to indicate purity and neutral pH: water with high resistivity (18.2 MΩ.cm at 25 °C) ensures that the pH is close to 7.0.1
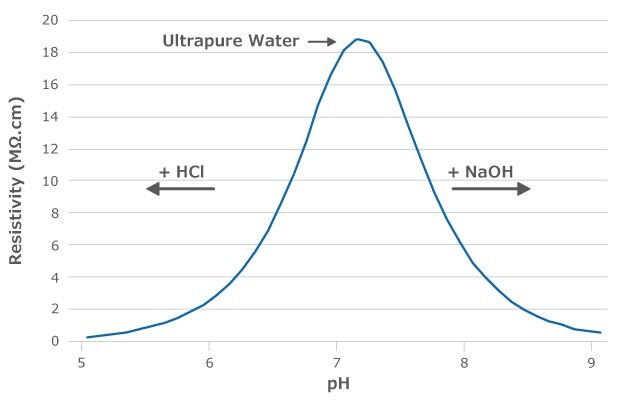
Figure 1.Theoretical resistivity values of water following the addition of hydrochloric acid (HCl) or sodium hydroxide (NaOH). The resistivity of ultrapure water is 18.2 MΩ.cm and the pH is 6.998.
Experiment: Impact of Buffer Dilution on pH Measurements
An experiment was designed to demonstrate that pure water delivered by a water system combining reverse osmosis and Elix® electrodeionization (such as a Milli-Q® IX Pure Water System) and ultrapure water delivered by a polishing unit (such as a Milli-Q® IQ 7000 Ultrapure Water System) could be used to prepare buffer solutions.1
Buffers are often prepared by diluting stock solutions. In this study, phosphate and PBS stock solutions were each diluted 10- and 100-fold with various water qualities and pH was measured. Results (Figure 2), show that the freshly produced pure and ultrapure water give identical results as bottled distilled water for buffer preparation.
The tenfold dilution of the 10× phosphate or PBS solution resulted in a pH close to the expected value of 7.4. Further diluting phosphate buffers with water resulted in pH increases due to changes in ionic strength and buffer capacity. For each buffer, the pH changes were similar with all three types of water.
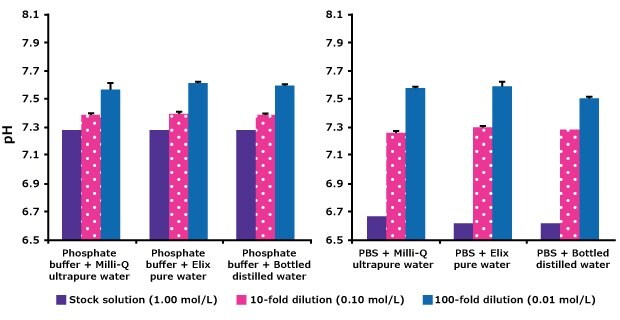
Figure 2.Effect on pH of diluting phosphate and PBS stock solutions with different types of water. Milli-Q®ultrapure water: resistivity 18.2 MΩ.cm, TOC < 5 ppb, bacteria 1 < cfu/mL. Elix® pure water: resistivity > 5 MΩ.cm, TOC < 30 ppb (measured in-line) and bacteria < 10 cfu/mL and bottled distilled water.
It should be remembered that temperature also has an impact on the pH value. Preparing buffers with very cold solutions may result in differences in pH of 0.1.
Lab Water Solutions for Measuring pH and Preparing Buffers
Water quality defined as pure water or Type 2 water is usually recommended to measure pH. When preparing buffers, the selection of water quality will be dependent on the purpose of the buffer solution: if it is meant for use in sensitive applications (cell culture, molecular biology, etc.) then Type 1 or ultrapure water would typically be preferred.
A range of water system solutions adapted to the needs of scientists preparing buffers and measuring pH is available.
Select and configure your optimal water purification system for your laboratory and its applications or request support from a lab water solution expert.
References
To continue reading please sign in or create an account.
Don't Have An Account?