Carcinogenesis and Epigenetics
Cancer research has revealed that the classical model of carcinogenesis, a three-step process consisting of initiation, promotion, and progression, is not complete. The expansion of the carcinogenesis model into a multi-mechanistic process occurring for an extended period has been supported by experimental studies concerning cancer stem cells, gap junction intercellular communication, and 3D culture techniques. Prior carcinogenicity and genotoxicity data collection coupled with current research provides a foundation for understanding the intricate carcinogenic process characterized by both mutagenic and epigenetic facets.
Classical Theory of Carcinogenesis, Initiators, and Tumor Promotors
The classical model of carcinogenesis (Figure 1) begins with initiation during which exposure to a carcinogen results in sequential genetic change to a single cell. Initiation proceeds to the promotion phase where additional carcinogenic exposure results in enhanced cell division.1,2 Carcinogenic “initiators”, from radiation (i.e., UV and X-ray) and chemical sources, combined with promoters are used in experimental models to induce tumor formation. Genotoxic initiators mutate cellular DNA using several mechanisms including alkylation, cytochrome P450 metabolism, or intracellular reactive oxygen species production. If the DNA damage to the cell is not adequately repaired and the cell becomes neither senescent nor apoptotic, the somatic DNA mutation event may be followed by promoter assisted cell proliferation and malignant tumor formation during the progression phase.
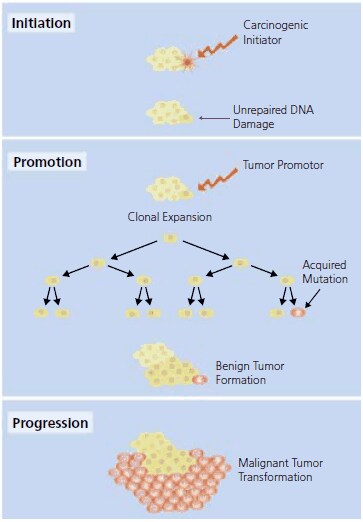
Figure 1.The classical model of carcinogensis proceedes from initiation to promotion and then evolves to progression. See text for further description.
In the experimental model of carcinogenesis, exposure to a carcinogenic initiator is followed by the repeated application of a non-mutagenic carcinogenic promoter to enhance cell proliferation. Specific hormones, drugs, infectious agents, and chemical carcinogens have been identified as tumor promoters in animal models. Chemical carcinogens that are commonly used to promote tumor formation in experimental models are found on same page, Tumor Promotors.
Tumors primarily arise from epithelial cells that form the lining of a cavity or duct. In the experimental model, the exposure of the epithelium to an initiator and promoter is followed by the progression phase, where the accrual of additional genetic mutations, oncogene expression, and chromosomal DNA exchange leads to carcinomas. Progression involves the transformation of the proliferating cells to an increasingly malignant growth state. Accrued genetic mutations are required for transformation of healthy epithelial cells into a carcinoma exhibiting abnormal growth, survival, and invasion properties. The mutations alter the function of the proteins encoded by growth-regulating genes (i.e., oncogenes and tumor suppressor genes) and ultimately influence unregulated cell proliferation.3
Carcinoma is the most common form of human cancer and arises in a multitude of epithelial cell layers found in the mouth, esophagus, intestines, stomach, mammary gland, pancreas, skin, lung, liver, ovary, gallbladder, and urinary bladder. Chemical carcinogens frequently used in an applied research setting to initiate mutagenic damage for carcinoma research are found on same page, Carcinogens.
Genotoxicity Data and Testing
The non-initiating carcinogens promote tumor formation via non-genetic, mitogenic, or cytotoxic mechanisms. In the experimental model of carcinogenesis, tumor promoters drive the formation of daughter cell populations from a common progenitor cell (i.e., clonal expansion). Clonal expansion is key to the cellular acquisition of an additional mutation; the clonal descendents must become so numerous that a mutation with a low probability of occurance arises within the expanded clonal cell population. The promotion phase of carcinogenesis results in a high tumor yield and acquisition of additional mutations through clonal expansion and cell replication.4 Additionally, tumor progression is driven during promoter-activated, repeated cell division as a result of the following events3:
1) Formation of mutant DNA sequences as a result of DNA miscopy
2) Mobilization of tumor suppressor genes during mitotic recombination and faulty chromosomal segregation
3) Shortening of the telomeric DNA in stem cells
The study of environmental carcinogen mutagenicity and the resultant genotoxicity-based evaluation of cancer risk has been a significant focus of cancer research since the development of the Ames’ test by Bruce Ames in 1975. The Ames test for mutagenicity is performed by combining a carcinogenic test compound with rat liver homogenate. This metabolically activated test sample is added to a Petri dish containing a special Salmonella strain unable to grow without supplemental histidine. Only bacteria that are mutated to a histidine-independent genotype will grow. The incidence of colony growth correlates to the mutagenicity of the test compound.
Since the introduction of the Ames test, other techniques have been developed to gauge the ability of a genotoxic agent to illicit damage on DNA and chromosomes. These techniques are used to monitor increases in:
1) Mutation, chromosomal aberration, or aneuploidy
2) DNA adducts or interference with repair of DNA damage
3) Nonspecific DNA or chromosomal damage
Genotoxicity testing data reported in the United States Environmental Protection Agency’s peer-reviewed online database, GENE-TOX, is derived from an assortment of in vitro and in vivo assays using various prokaryotic, eukaryotic, and mammalian species. GENE-TOX is accessible, free of charge, via TOXNET (see Table 1 for GENE-TOX and other resources).
| Carcinogenic Resource | Information Available | Web Site |
|---|---|---|
| TOXNET: CCRIS | NCI maintained data bank with Over, 9,000 chemical records with carcinogenicity. Mutagenicity, tumor promotion, and tumor inhibition test results. | http://toxnet.nlm.nih.gov |
| TOXNET: GENE-TOX | Database developed by the U.S. EPA with genetic toxicology (mutagenicity) test data from scientific literature for 3000 + chemicals. | http://toxnet.nlm.nih.gov |
| Carcinogenic Potency Database | Database; with the results of animal cancer tests on 1,547 chemicals. Prepared by the University of California Berkeley and the Lawrence Berkeley National Laboratory. | http://potency.berkeley.edul.index.html |
It has been argued that the importance placed on the mutagenic data derived from in vitro assays and rodent models over the past thirty years has not only encouraged the expectation that carcinogens deemed mutagenic are cancer-inducing in humans but also reinforced the “carcinogen equals mutagen” assumption. This could be considered a narrow viewpoint since the complete process is not well represented by genotoxicity analysis. Carcinogenesis is deemed an intricate process occurring over an extended time period and dependent upon epigenetic events induced by non-genotoxic carcinogens.5,6
Epigenetics and Current Techniques
Carcinogens that are non-genotoxic, non-apoptotic, and non-cytotoxic to the cell may still contribute to carcinogenesis in an epigenetic manner, by directly affecting gene expression during transcription, translation, and post-translational events. The epigenetic mechanisms used by this special class of carcinogens yields heritable or non-heritable changes to the methylation and acetylation patterns of DNA and histones.7,8 There are many nongenotoxic agents that induce epigenetic changes in both proliferating and non-proliferating cells. For example, arsenic, an established human carcinogen, does not initiate mutagenic damage but rather contributes to carcinogenesis by increasing reactive oxygen species (ROS) production.9 Arsenic-assisted ROS production, specifically reported in a study of pulmonary tissue, resulted in altered gene expression with oxidative damage identified as the key contributor.10 Furthermore, Li, et al. demonstrated that arsenic activates stress gene expression of γ-glutamylcysteine synthetase in cultured lung epithelial cells.11 Arsenic, DDT,12 phenobarbital,13 saccharin,14 and peroxisome proliferators15 have been experimentally shown to contribute to carcinogenesis without a genotoxic contribution.6
Aberrations in gene expression, such as those induced by arsenic, result in an imbalance between apoptosis, proliferation, and differentiation leading to cancer or other disease states.16 Altered gene expression is usually preceded by modifications in normal cell signaling during translation and intercellular signaling within tissues.
A multi-cellular organism’s homeostatic balance is dependent on intra- and inter-cellular signaling conversations. Carcinogens have been implicated for their epigenetic contribution to altered gene expression in gap junctional intercellular communication (GJIC). Cells connected via gap junctions use GJIC channels to maintain homeostasis.17 Carcinogenic exposure may disrupt the organism’s homeostatic balance by inhibiting GJIC, leading to the “awakening” of a quiescent cell that is ordinarily suppressed through contact inhibition.5
Gap junctions are specialized cell membrane domains consisting of aggregations of intercellular channels that directly connect the cytoplasm of adjacent cells.18 Gap junctions coordinate cellular and organ function in tissues and are involved in synchronization of cellular physiological activities, growth control, and developmental regulation. The gap junction channels allow intercellular exchange of ions, nucleotides, and small molecules between adjacent cells. Unlike other membrane channels, intercellular channels span two apposed plasma membranes and require the contribution of hemi-channels, called connexons, from both participating cells.19 Gap junction protein levels change in response to disruption of tissue architecture.20 Reduction or alteration in the levels or types of connexin expressed in various cell types correlates with tumor progression and metastasis.
With the expression and function of connexin genes being highly relevant to the cell growth cycle, it is not surprising that a high percentage of malignant cells are marked by dysfunctional GJIC and that tumor cells exhibit altered connexin localization, contributing to a lack of cell adhesion functionality.9 Non-genotoxic promoters can block GJIC at non-cytotoxic levels by blocking the “contact-inhibition” between cells and allowing them to proliferate.21 This evidence is supportive of the concept that GJIC is the cellular mechanism responsible for tumor promotion and the ability of “initiated” cells to escape suppression by intercellular communication.
Compounds previously assumed to initiate mutation to oncogenes in the tumors of the chemically exposed animal have been revisited to aid in the understanding of their possible epigenetic effect. Cha, et al. reported that Ha-ras-1 oncogene mutations in mammary epithelial cells do not contribute to initiation of spontaneous mammary tumorigenesis in rats and in a second study reported that N-nitroso-N-methylurea-induced rat mammary tumors arise from cells with preexisting oncogenic Ha-ras-1 gene mutations.22,23 Additionally, Brookes et al.24 and Mass and Austin25 previously showed that 7,12-dimethylbenz[a]anthracene (DMBA) did not mutate the Ki-ras and Ha-ras oncogene of the DMBA-transformed cells.17
Recent polycyclic aromatic hydrocarbon (PAH) research also exemplifies the re-evaluation of carcinogenic substances as the key mutagenic players in carcinogenesis. Since being identified as mutagenic, high molecular weight PAHs found in tobacco smoke (e.g., benzo[a]pyrene) have been the focus of tobaccorelated cancer research. However, the high molecular weight PAHs represent a substantially lower percentage of the chemical constituent profile of tobacco smoke. Taking into account the facts that (a) the lower molecular weight PAHs have demonstrated high carcinogenicity when applied after benzo[a]pyrene treatment,26 (b) tobacco smoke is a strong promoter and weak complete carcinogen,27-30 and (c) that former smokers face the same low risk of tobacco-related cancer development as a non-smoker,31 one may infer that low molecular weight PAHs contribute in a non-genotoxic manner to tobacco-related cancer development.17 Epigenetic-related toxicity research on the structure-function relationship of PAHs and GJIC inhibition is ongoing.6,32,33
Rosenkranz, et al. conducted a comprehensive structure-activity relationship study to uncover correlations between GJIC inhibition and molecules with varied toxicological properties. The authors suggested that GJIC inhibition is not linked to genotoxic mechanisms, but instead GJIC is associated with the balance of cell cycle functionality: proliferation, cell differentiation, and apoptosis.34
Similarly, Trosko et al. championed the importance of epigenetic endpoint testing in the mechanistic study of cell cycle function, methylation change, and cell-cell communication using normal human stem cell 3D in vitro assay techniques.8,35 Rather than conducting endpoint testing at the genomic level with DNA microarray gene expression analysis, some scientists recommend that the experimental focus should be at the tissue level. 3D culture techniques have been used to examine the role of interactions between epithelial cells and the extracellular matrix (ECM) in regards to apoptotic cell behavior.36 Since the 3D culture system is currently the best in vitro representation of the in vivo cellular microenvironment these techniques are also being applied in the study of epigenetic events induced by the stem cell microenvironment.37
As a thorough understanding of the mechanistic operation of carcinogenesis is elucidated and combined with supplemental data from prior research, improved prevention guidelines and therapeutic treatments will be developed. The emphasis on specific anticancer agents, such as bioactive nutrients, will increase as the significance of their interactions is validated by information provided through the continued study of carcinogenesis.6
References
To continue reading please sign in or create an account.
Don't Have An Account?