Sepharose® High Performance: Purification with High Resolution
- Use Sepharose® High Performance for intermediate or polishing steps that require high resolution (recommended flows up to 300 cm/h). Use when SOURCE™ media do not offer the required selectivity.
- Use Sepharose® High Performance (34 μm particle size) HIC media for capture or scale-up when selectivity is satisfactory, high resolution is a priority and if lower flow rates (to compensate for a higher back pressure) are acceptable.
- Run Sepharose® High Performance columns on systems such as ÄKTAdesign™, FPLC System and HPLC. Appendix 3 gives guidance on how to select the most suitable ÄKTAdesign™ system.
Sepharose® High Performance is based on a matrix of particles (mean size 34 μm) made from 6% agarose and highly cross-linked for chemical and physical stability. The small particle size facilitates fast binding and dissociation even at high sample loads and flow rates. In combination with the correct selectivity, high-resolution separations can be achieved. Particle size and bed volumes remain stable, despite changes in salt concentration, to ensure fast separations at high flow rates. Ligands are coupled via a chemically stable O-ether linkage (Figure 1).
Sepharose® High Performance can be used for group separations, sample concentration or capture steps. However, these separations should be limited to reasonably clean samples to avoid the risk of blocking the column filter (34 μm particles requires the use of finer column filters).

Figure 1. Ligands are coupled to the Sepharose® High Performance matrix via uncharged, chemically stable O-ether linkages.
Purification Options

Figure 2. Phenyl Sepharose® High Performance is available prepacked in HiTrap™ or HiLoad™ columns and in media packs.
* Recommendations are for separations at room temperature in aqueous buffers. See Appendix 3 to convert linear flow (cm/hour) to volumetric flow rates (mL/min) and vice versa. Note that final working flow will depend also on factors such as column size and bed height, sample characteristics and loading conditions, the equipment used and the back pressure that the equipment can withstand.
† Maximum operating back pressure refers to the pressure above which the medium begins to compress.
‡ Refer to page 19 for more details about the influence of ligand density.
It is important to note that the binding capacity of a HIC medium is highly dependent on the properties of the target protein and contaminants, the selectivity of the medium and the binding conditions.
Capacity must be determined empirically during media screening and method development.
Use the HiTrap™ HIC Selection Kit, comprising six prepacked HiTrap™ 1 mL columns containing media with different hydrophobic characteristics, to rapidly screen for the most suitable medium for a specific application (Figure 3). Select the medium that gives the best selectivity, resolution and loading capacity at the lowest salt concentration.
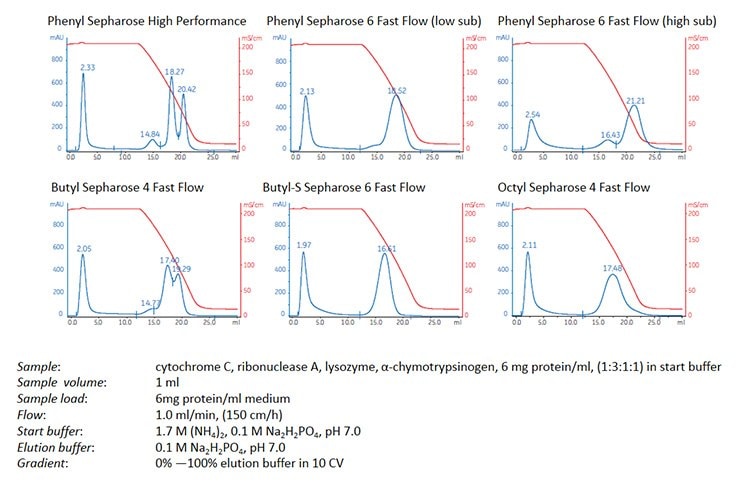
Figure 3. Comparison of the different selectivity characteristics in a HiTrap™ HIC Selection Kit.
Use prepacked HiTrap™ columns (1 mL or 5 mL) for method scouting, group separations, smallscale purification, sample concentration or clean-up. When increased capacity is required, use prepacked columns HiLoad 16/10 Phenyl Sepharose® HP (20 mL) or HiLoad 26/10 Phenyl Sepharose® HP (53 mL).
Phenyl Sepharose® High Performance and Phenyl Sepharose® 6 Fast Flow (low sub) usually exhibit similar hydrophobic properties. For capture or intermediate purification, Phenyl Sepharose® 6 Fast Flow (low sub) may give similar resolution at higher flow rates.
Butyl Sepharose® High Performance is available only as a Custom Designed Product (see page 50). The butyl ligand offers an alternative selectivity to Phenyl Sepharose® High Performance.
Select a production column such as FineLINE for larger volumes.
Purification examples
Media Screening: Development of a Monoclonal Antibody Purification
The most common contaminants of monoclonal antibody preparations are albumin and transferrin. However, since most monoclonal antibodies are more hydrophobic than these contaminants, HIC can be used to bind the antibody as the contaminants wash through the column. Figure 4 shows an example of screening using columns from the HiTrap™ HIC Selection Kit in order to select a HIC medium that could offer the best selectivity and resolution for purification of a monoclonal IgG. Phenyl Sepharose® High Performance produced a well-resolved peak containing IgG (note that this peak is not necessarily the pure IgG, but may represent a series of components with similar hydrophobicity to the IgG).

Figure 4. Media screening for monoclonal antibody purification.
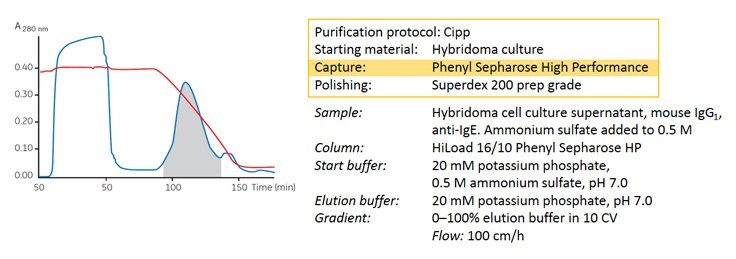
Figure 5. Concentration and purification of a monoclonal antibody from hybridoma cell culture.
Figure 5 shows an example of high selectivity used in a capture step. The goal of the purification was to produce a monoclonal antibody of sufficient purity for in vitro diagnostic use. The mouse IgG1 anti-IgE, produced in a hybridoma cell culture, bound very strongly to Phenyl Sepharose® High Performance, and most fetal calf serum proteins passed through the column. No intermediate step was required as the capture step gave a purity level >95%. The sample was concentrated into a small volume that could be transferred directly to a polishing step.
Intermediate Purification: Recombinant HIV Reverse Transcriptase
In this example an E. coli lysate was subjected to ammonium sulfate precipitation (see Appendix 1) followed by a capture step using ion exchange chromatography. After addition of ammonium sulfate, HIC was used to concentrate and further purify the sample using a gradient elution prior to a final polishing step using another ion exchange medium.
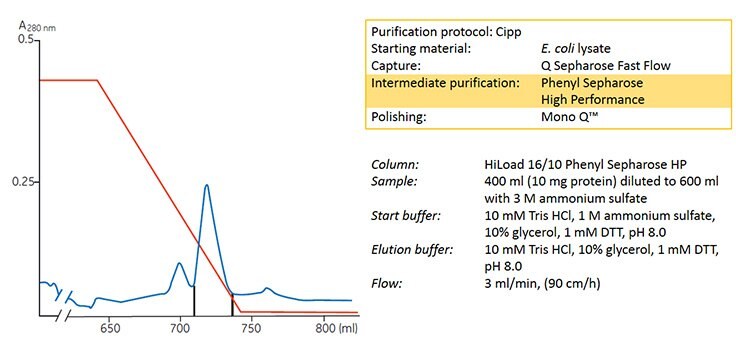
Figure 6. Concentration and purification of HIV reverse transcriptase on HiLoad 16/10 Phenyl Sepharose® HP.
To continue reading please sign in or create an account.
Don't Have An Account?