UHPLC Analysis of Diclofenac in Gel using a Monolithic Silica Column
A comparison to a sub-2 µm fully porous particulate (FPP) hybrid silica column with UV & MS detection
![Diclofenac Sodium Gel Chemical structure of diclofenac sodium (2-[(2,6-Dichlorophenyl) amino] benzeneacetic acid sodium salt, diclofenac sodium salt)](/deepweb/assets/sigmaaldrich/marketing/global/images/technical-documents/articles/analytical-chemistry/small-molecule-hplc/diclofenac-sodium-gel/diclofenac-sodium-gel.jpg)
Diclofenac sodium
Introduction
Diclofenac is a nonsteroidal anti-inflammatory drug (NSAID) used to treat pain and inflammatory diseases. Diclofenac was patented in 1965 and came into medical use in the United States in 1988. It is available as a sodium or potassium salt. This report focuses on the analysis of diclofenac sodium salt in a gel under UHPLC conditions. Matrix-rich formulations such as gel, typically require extensive sample preparation in particular when using sub-2 µm UHPLC columns. In this work, the sample preparation was kept simple as extensive sample preparation significantly contributes to the time and cost spent per analysis.
A Chromolith® HighResolution RP-18 endcapped column 100 x 2 mm I.D. is compared with a fully porous particulate (FPP) 1.7 µm hybrid silica C18 column 100 x 2.1 mm I.D. from a competitor using HPLC-MS and HPLC-UV methods. For both columns, a stability test was performed with repeated sample overloading in order to stress both columns to a maximum.
Experimental Method: Diclofenac Sodium in Gel with MS detection
- HPLC-MS analysis of diclofenac sodium standard solutions using Chromolith® HighResolution
- HPLC-MS analysis of diclofenac sodium standard solutions using FPP 1.7 μm hybrid silica column
- HPLC-MS analysis of diclofenac gel sample solutions using Chromolith® HighResolution
- HPLC-MS analysis of diclofenac gel sample solutions using FPP 1.7 μm hybrid silica column
| HPLC-MS Conditions | |
|---|---|
| Column 1: | Chromolith® HighResolution RP-18e 100 x 2.0 mm I.D. (1.52322) |
| Column 2: | FPP Competitor column (hybrid silica C18 1.7 µm, 100 x 2.1 mm I.D.) |
| Mobile phase: | [A] Water + 0.1% formic acid [B] Methanol + 0.1% formic acid |
| Isocratic: | A/B 34/66 (v/v) |
| Flow Rate: | 200 µL/min for 2.0 mm I.D. and 221 µL/min for 2.1 mm I.D. |
| Temperature: | 30 °C |
| Pressure Drop: | 86 bar (1247 psi) for Chromolith® HighResolution 468 bar (6786 psi) for FPP 1.7 µm hybrid silica column |
| Injection Volume: | 1 µL |
| Detection: | Shimadzu LCMS 20; ESI+ |
| Standard solutions (50 µg/mL) | 0.05% (w/v) of diclofenac sodium was dissolved in methanol, then further diluted taking 1 volume of the resulting solution to 10 volumes using the mobile phase. |
| Sample Solution | A quantity of 6 g of the gel containing 5 mg/g of diclofenac sodium was shaken with 50 mL acetone for 10 minutes. The solution was filtered, and the filtrate evaporated to dryness under a gentle nitrogen flow. The residues was dissolved in 100 mL of a mixture of 40 volumes of water and 60 volumes of methanol, 1 volume of this solution was diluted to 10 volumes with the mobile phase and then filtered through a glass fibre filter. |
| LC-MS settings | |
|---|---|
| Detection | Shimadzu LCMS 20; ESI+ |
| DL temperature | 250 °C |
| Heating block temperature | 200 °C |
| Interface voltage | 4.5 V |
| Detector voltage | 0.9 V |
| Dry gas | 1.5 L/h |

Figure 1.LC-MS Data on separation of diclofenac sodium standard solution on
Chromolith® HighResolution RP-18 endcapped 100 x 2 mm I.D. column.
1. Specificity: Inject a standard solution of diclofenac sodium and determine the retention time and content of the desired analyte with MS detection.
No. | Compound | Retention Time (min) | Quantitation ion m/z | Area (Counts) |
|---|---|---|---|---|
1 | Diclofenac sodium | 5.3 | 296 | 378161 |
2. Sample repeatability of diclofenac sodium gel with MS detection
| Samples | Area (Counts) |
|---|---|
| STD 1 | 265764 |
| STD 2 | 264397 |
| STD 3 | 265396 |
| STD 4 | 265121 |
| STD 5 | 264351 |
| Mean | 265006 |
| Standard Deviation | 620 |
| (%) RSD | 0.2 |
3. LOD & LOQ diclofenac sodium with MS detection
| Conc. (µg/mL) | Mean Area (Counts) |
|---|---|
| 5 | 42097 |
| 10 | 82103 |
| 25 | 207676 |
| 50 | 410393 |
| 75 | 614103 |
| 100 | 817291 |
| STEYEX | 1234.8 |
| Slope | 8161.7 |
| LOD (µg/mL) | 0.5 |
| LOQ (µg/mL) | 1.5 |
4. Linearity diclofenac sodium with MS detection
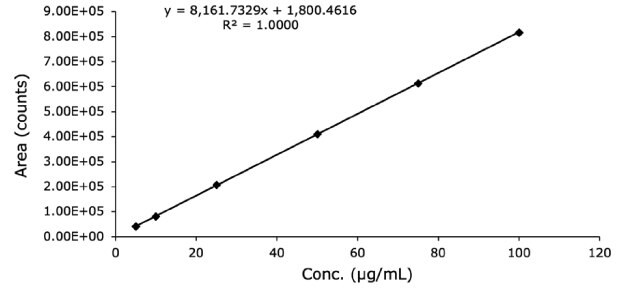
Figure 2.Linearity data with LC-MS on separation of diclofenac sodium standard solution on Chromolith® HighResolution RP-18 endcapped 100 x 2 mm I.D. column.

Figure 3.LC-MS Data on separation of diclofenac sodium standard solution on FPP 1.7 μm hybrid silica C18 column 100 x 2.1 mm I.D.
1. Specificity: Inject a standard solution of diclofenac sodium and determine the retention time and content of the desired analyte with MS detection.
| No. | Compound | Retention Time (min) | Quantitation ion m/z | Area (Counts) |
|---|---|---|---|---|
| 1 | Diclofenac sodium | 9.9 | 296 | 376893 |
2. Sample repeatability of diclofenac sodium gel with MS detection
| Sample | Area (Counts) |
|---|---|
| STD 1 | 263385 |
| STD 2 | 261452 |
| STD 3 | 261853 |
| STD 4 | 262274 |
| STD 5 | 261761 |
| Mean | 262145 |
| Standard Deviation | 753 |
| (%) RSD | 0.3 |
3. LOD & LOQ diclofenac sodium with MS detection
| Conc. (µg/mL) | Mean Area (Counts) |
|---|---|
| 5 | 42874 |
| 10 | 83303 |
| 25 | 209917 |
| 50 | 410865 |
| 75 | 614276 |
| 100 | 815050 |
| STEYEX | 2102.7 |
| Slope | 8129.5 |
| LOD (µg/mL) | 0.9 |
| LOQ (µg/mL) | 2.6 |
4. Linearity diclofenac sodium with MS detection

Figure 4.Linearity data with LC-MS on separation of diclofenac sodium standard solution on FPP 1.7 μm hybrid silica C18 column 100 x 2.1 mm I.D.

Figure 5.LC-MS Data on separation of diclofenac gel sample solution on Chromolith® HighResolution RP-18 endcapped 100 x 2 mm I.D. column.
Chromatographic data - sample solution (Figure 5.)
| No. | Compound | Retention Time (min) | Quantitation ion m/z | Area (Counts) |
|---|---|---|---|---|
| 1 | Diclofenac sodium | 5.3 | 296 | 264379 |


Figure 6.LC-MS Data on separation of diclofenac gel sample solution on FPP 1.7 μm hybrid silica C18 column 100 x 2.1 mm I.D.
Chromatographic data - sample solution (Figure 6.)
| No. | Compound | Retention Time (min) | Quantitation ion m/z | Area (Counts) |
|---|---|---|---|---|
| 1 | Diclofenac sodium | 9.9 | 296 | 261452 |

Experimental Method: Diclofenac Sodium in Gel with UV Detection
- HPLC-UV analysis of diclofenac sodium standard solutions on Chromolith® HighResolution
- HPLC-UV analysis of diclofenac sodium standard solutions on FPP 1.7 μm hybrid silica column
- HPLC-UV analysis of diclofenac gel sample solutions using Chromolith® HighResolution
- HPLC-UV analysis of diclofenac gel sample solutions using FPP 1.7 μm hybrid silica column
| HPLC-UV Conditions | |
|---|---|
| Column 1: | Chromolith® HighResolution RP-18e 100 x 2.0 mm I.D. |
| Column 2: | FPP Competitor column (hybrid silica C18 1.7 µm, 100 x 2.1 mm I.D.) |
| Mobile phase: | [A] Water + 0.1% formic acid [B] Methanol + 0.1% formic acid |
| Isocratic: | A/B 34/66 (v/v) |
| Flow Rate: | 200 µL/min for 2.0 mm I.D. and 221 µL/min for 2.1 mm I.D. |
| Temperature: | 30 °C |
| Pressure Drop: | 86 bar (1247 psi) for Chromolith® HighResolution 468 bar (6786 psi) for FPP 1.7 µm hybrid silica column |
| Injection Volume: | 1 µL |
| Detection: | PDA @254 nm |
| Standard solutions (50 µg/mL) | 0.05% (w/v) of diclofenac sodium was dissolved in methanol, then further diluted taking 1 volume of the resulting solution to 10 volumes using the mobile phase. |
| Sample Solution | A quantity of 6 g of the gel containing 5 mg/g of diclofenac sodium was shaken with 50 mL acetone for 10 minutes. The solution was filtered, and the filtrate evaporated to dryness under a gentle nitrogen flow. The residue was dissolved in 100 mL of a mixture of 40 volumes of water and 60 volumes of methanol, 1 volume of this solution was diluted to 10 volumes with the mobile phase and then filtered through a glass fiber filter. |

Figure 7.Blank run UV data on Chromolith® HighResolution RP-18 endcapped 100 x 2 mm I.D. column.
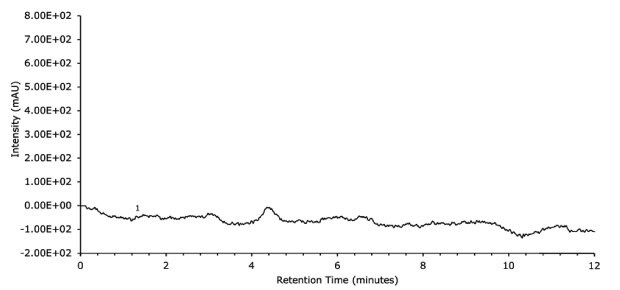
Figure 8.Blank run UV data on FPP 1.7 μm hybrid silica C18 column 100 x 2.1 mm I.D.

Figure 9.UV Data on separation of diclofenac sodium standard solution on Chromolith® HighResolution RP-18 endcapped 100 x 2 mm I.D. column.
1. Specificity: Inject a standard solution of diclofenac sodium and determine the retention time and content of the desired analyte with UV detection.
| No. | Compound | Retention Time (min) | S/N | Area (Counts) | Tailing Factor |
|---|---|---|---|---|---|
| 1 | t0 | 1.5 | |||
| 2 | Diclofenac sodium | 5.1 | 1060.2 | 296274 | 1.2 |
2. Sample repeatability of diclofenac sodium gel in area (counts) with UV detection
| Samples | Area (Counts) |
|---|---|
| STD 1 | 167858 |
| STD 2 | 167475 |
| STD 3 | 167718 |
| STD 4 | 167316 |
| STD 5 | 167425 |
| Mean | 167558 |
| Standard Deviation | 223 |
| (%) RSD | 0.1 |
3. LOD & LOQ diclofenac sodium with UV detection
| Conc. (µg/mL) | Mean Area (Counts) |
|---|---|
| 5 | 24882 |
| 10 | 56262 |
| 25 | 146502 |
| 50 | 295264 |
| 75 | 439453 |
| 100 | 583866 |
| STEYEX | 2464.6 |
| Slope | 5880.3 |
| LOD (µg/mL) | 1.4 |
| LOQ (µg/mL) | 4.2 |
4. Linearity diclofenac sodium with UV detection

Figure 10.Linearity data with UV on separation of diclofenac sodium standard solution on Chromolith® HighResolution RP-18 endcapped 100 x 2 mm I.D. column.
HPLC-UV analysis of diclofenac sodium standard solutions on FPP 1.7 μm hybrid silica column

Figure 11.UV Data on separation of diclofenac sodium standard solution on FPP 1.7 μm hybrid silica C18 column 100 x 2.1 mm I.D.
1. Specificity: Inject a standard solution of diclofenac sodium and determine the retention time and content of the desired analyte with UV detection.
| No. | Compound | Retention Time (min) | S/N | Area (Counts) | Tailing Factor |
|---|---|---|---|---|---|
| 1 | t0 | 1.4 | |||
| 2 | Diclofenac sodium | 9.7 | 1558.2 | 308174 | 1.1 |
2. Sample repeatability of diclofenac sodium gel with UV detection
| Samples | Area (Counts) |
|---|---|
| STD 1 | 174899 |
| STD 2 | 175642 |
| STD 3 | 174113 |
| STD 4 | 174814 |
| STD 5 | 175721 |
| Mean | 175038 |
| Standard Deviation | 663 |
| (%) RSD | 0.4 |
3. LOD & LOQ diclofenac sodium with UV detection
| Conc. (µg/mL) | Mean Area (Counts) |
|---|---|
| 5 | 24986 |
| 10 | 56240 |
| 25 | 151867 |
| 50 | 308038 |
| 75 | 462262 |
| 100 | 612579 |
| STEYEX | 2438.2 |
| Slope | 6197.5 |
| LOD (µg/mL) | 1.3 |
| LOQ (µg/mL) | 3.9 |
4. Linearity diclofenac sodium with UV detection
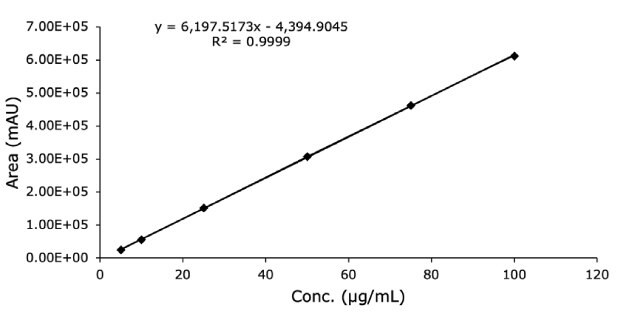
Figure 12.Linearity data with UV on separation of diclofenac sodium standard solution on FPP 1.7 μm hybrid silica column from competitor 100 x 2.1 mm I.D.
HPLC-UV analysis of diclofenac gel sample solutions using Chromolith® HighResolution

Figure 13.UV Data on separation of diclofenac gel sample solution on Chromolith® High Resolution RP-18 endcapped 100 x 2 mm I.D. column.
Chromatographic data - sample solution (Figure 13.)
| No. | Compound | Retention Time (min) | S/N | Area (Counts) | Tailing Factor |
|---|---|---|---|---|---|
| 1 | t0 | 1.5 | |||
| 2 | Diclofenac sodium | 5.1 | 916.8 | 167518 | 1.2 |

HPLC-UV analysis of diclofenac gel sample solutions using FPP 1.7 μm hybrid silica column
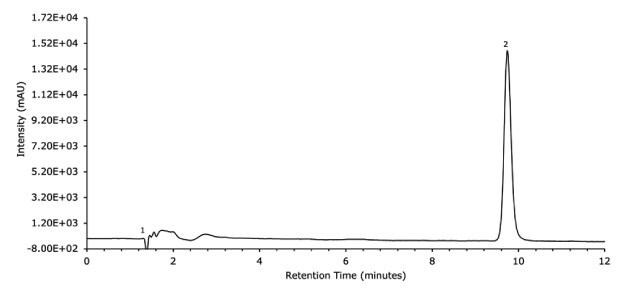
Figure 14.UV Data on separation of diclofenac gel sample solution on FPP 1.7 μm hybrid silica C18 column 100 x 2.1 mm I.D.
Chromatographic data - sample solution (Figure 14.)
| No. | Compound | Retention Time (min) | S/N | Area (Counts) | Tailing Factor |
|---|---|---|---|---|---|
| 1 | t0 | 1.4 | |||
| 2 | Diclofenac sodium | 9.7 | 722.41 | 174618 | 1.1 |

Stability Test – Chromolith® monolithic silica column v/s FPP µm hybrid silica column
The stability test was performed to demonstrate the resistance to column clogging rep. matrix-tolerance and stability of a monolithic silica column in comparison to a 1.7 μm fully porous particulate (FPP) column which is typically used in UHPLC applications. Both columns have been treated the same way with frequent overloading to stress both columns to a maximum.
Both columns were tested with three 1 μL injections after stressing the columns 10 times with 10 μL injections. No pre-columns were used for this test.
| Experimental Conditions for Stability Test | |
|---|---|
| Column 1: | Chromolith® HighResolution RP-18e 100 x 2.0 mm I.D. (1.52322) |
| Column 2: | FPP Competitor column (hybrid silica C18 1.7 µm, 100 x 2.1 mm I.D.) |
| Mobile phase: | [A] Water [B] Methanol |
| Isocratic: | A/B 35/65 (v/v) |
| Flow Rate: | 200 µL/min for 2.0 mm I.D. and 221 µL/min for 2.1 mm I.D. (alignment of linear flow conditions - 2.0 mm I.D. for monolithic vs. 2.1 mm I.D. for FPP) |
| Temperature: | Ambient |
| Injection Volume: | 3 times 1 μL; 10 times 10 μL; 3 times 1 μL; 10 times 10 μL etc. |
| Detection: | UV @254 nm |
| Sample Solution | A quantity of 6 g of the gel containing 5 mg/g of diclofenac sodium was shaken with 50 mL acetone for 10 minutes. The solution was filtered, and the filtrate evaporated to dryness under a gentle nitrogen flow. The residue was dissolved in 100 mL of a mixture of 40 volumes of water and 60 volumes of methanol, 1 volume of this solution was diluted to 10 volumes with the mobile phase and then filtered through a glass fiber filter (Whatmann GF/C). |
Column backpressure / Injection volume
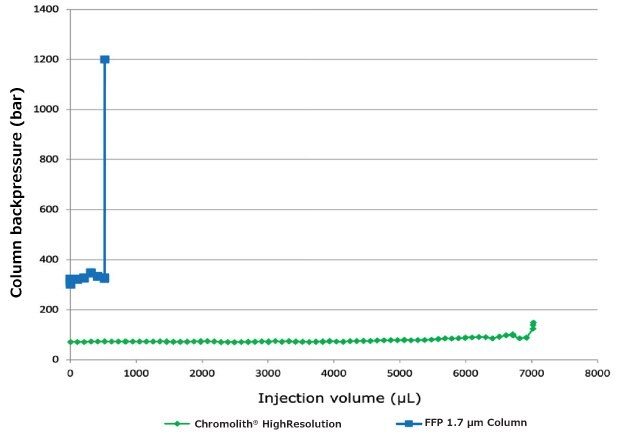
Figure 15.Stability test data on Chromolith®HighResolution RP-18 endcapped 100 x 2 mm I.D. column and FPP 1.7 μm hybrid silica column from competitor 100 x 2.1 mm I.D. Data points with 1 mL injections were used for measurement.
Conclusion
It was shown, that a Chromolith® HighResolution RP-18e 100 x 2 mm I.D. column can be utilized for the determination of Diclofenac sodium in a gel with a typical UHPLC instrument. The resulting LOD and LOQ values are comparable to the values of the fully porous particulate (FPP) 1.7 µm hybrid silica C18 column 100 x 2.1 mm I.D. with UV and MS detection. The limit of detection (LOD) with UV detection was 1.4 µg/mL and the limit of quantitation (LOQ) with UV detection was 4.2 µg/mL for the Chromolith® HighResolution RP-18e 100 x 2 mm column. By using MS detection, the sensitivity was improved to 0.5 µg/mL (LOD) and 1.5 µg/mL (LOQ). Due to its large macroporous structure, the Chromolith® monolithic silica column shows high permeability with significant lower column backpressure in comparison to the FPP 1.7 µm column. The bi-modal pore structure of monolithic columns also results in short analysis times. In addition, monolithic silica columns show an outstanding robustness and high matrix-tolerance. In a stability test, stressing the columns to the limit, the monolithic column was able to allow for 7000 µL of gel sample to be injected. Under same conditions the fully porous particulate (FPP) 1.7 μm hybrid silica C18 column 100 x 2.1 mm I.D. clogged after 520 µL total injected sample volume. As a consequence, the 1.7 µm FPP column will require an extended sample preparation in order to ensure a longer column lifetime. This results in significantly higher cost per sample and additional time spend per analysis.
Learn more about Chromolith® monolithic silica columns at SigmaAldrich.com/chromolith
To continue reading please sign in or create an account.
Don't Have An Account?