Performing High-Throughput Screening Using His MultiTrap™ HP and His MultiTrap™ FF 96-Well filter Plates
His MultiTrap™ HP and His MultiTrap™ FF are prepacked, disposable 96-well filter plates for reproducible, high-throughput screening of histidine-tagged recombinant protein expression. Typical applications are expression screening of different constructs, screening for solubility of proteins, and optimization of the conditions for small-scale parallel purification. The plates are prepacked with precharged Ni Sepharose® High Performance and Ni Sepharose® 6 Fast Flow, respectively.

Figure 3.6His MultiTrap™ HP and His MultiTrap™ FF
Each well of the prepacked His MultiTrap™ HP and His MultiTrap™ FF contains 500 µl of a 10% slurry of Ni Sepharose® High Performance or Ni Sepharose® 6 Fast Flow in storage solution (50 µl of medium in 20% ethanol) and has a capacity for purifying up to 1.0 mg and 0.8 mg of histidine-tagged protein, respectively. The plates are made of polypropylene and polyethylene.
Characteristics of the chromatography media and of His MultiTrap™ HP and His MultiTrap™ FF are listed in Appendix 1 (Characteristics of Ni Sepharose, Ni Sepharose® excel, TALON® Superflow, and uncharged IMAC Sepharose® products). The Ni2+-charged media are compatible with all commonly used aqueous buffers, reducing agents, denaturants, such as 6 M Gua-HCl and 8 M urea, and a range of other additives. Prepacked His MultiTrap™ HP and His MultiTrap™ FF plates provide well-to-well and plate-to-plate reproducibility in terms of yield and purity of eluted protein. Automated robotic systems can be used, as well as manual handling using centrifugation or vacuum pressure. The purification procedure can easily be scaled up because Ni Sepharose® is available in both larger prepacked formats and as lab packs. This allows screening using His MultiTrap™ plates followed by scale-up on a HisTrap™ 1 ml or 5 ml column using best conditions, which shortens optimization time.
Sample and buffer preparation
His MultiTrap™ HP: refer to Purification using Ni Sepharose® High Performance earlier in this chapter for a general procedure for sample and buffer preparation.
His MultiTrap™ FF: refer to Purification using Ni Sepharose® 6 Fast Flow earlier in this chapter for a general procedure for sample and buffer preparation.
After thorough cell disruption, it is possible to apply unclarified lysate directly to the wells without precentrifugation and/or filtration of the sample.
Apply the unclarified lysate to the wells directly after preparation, as the lysate may precipitate unless used immediately or frozen and thawed before use. Samples with precipitation may be sonicated to reduce clogging of the wells. Note that aging of the sample may reduce yields of the target protein.
Lysis with commercial kits could give large cell debris particles that may interfere with drainage of the wells during purification. This problem can be solved by centrifugation or filtration of the sample before adding it to the wells.
Centrifugation procedure for high-throughput screening
Preparing the 96-well filter plate
- Peel off the bottom seal from the 96-well filter plate. Be sure to hold the filter plate over a sink to accommodate any leakage of storage solution when removing the bottom seal.
- Hold the filter plate upside down and gently shake it to dislodge any medium adhering to the top seal. Return the filter plate to an upright position.
- Place the filter plate against the bench surface and peel off the top seal.
- Position the filter plate on top of a collection plate.
Note: Remember to change or empty the collection plate as necessary during the following steps. - Centrifuge the filter plate for 2 min at 500 × g to remove the ethanol storage solution from the medium.
- Add 500 µl of deionized water to each well. Centrifuge the plate for 2 min at 500 × g.
- Add 500 µl of binding buffer to each well to equilibrate the medium. Centrifuge for 2 min at 500 × g. Repeat once. The filter plate is now ready for use.
Blank run: Reducing agents may be used in sample and buffers. In such a case, perform a blank run by applying 500 µl of elution buffer/well before step 7. No reducing agent should be used in buffer during blank runs. Reequilibrate with binding buffer that includes reducing agent before sample application. Do not leave His MultiTrap™ plates with buffers that include reducing agents when not in use.
Centrifugation procedure
Do not apply more than 700 × g during centrifugation.
- Apply unclarified or clarified lysate (maximum 600 µl per well) to the wells of the filter plate and incubate for 3 min.
Note: If the yield of protein is too low, increase the incubation time and/or gently agitate the filter plate to suspend the medium in the sample solution. - Centrifuge the plate at 100 × g for 4 min or until all the wells are empty. Discard the flowthrough.
- Add 500 µl of binding buffer per well to wash out any unbound sample. Centrifuge at 500 × g for 2 min. Repeat once or until all unbound sample is removed.
Note: Removal of unbound material can be monitored as A280. For high purity, A280 should be < 0.1. - Add 200 µl of elution buffer per well and mix for 1 min.
Note: The volume of elution buffer can be varied (50 to 200 µl per well), depending on the concentration of target protein required. Volumes in the collection plate that are too small may give inaccurate absorbance values. - Change the collection plate and centrifuge at 500 × g for 2 min to collect the eluted protein. Repeat twice or until all the target protein has been eluted.
Note: The yield can usually be monitored for each fraction as A280 reading. If uncertain of required volume, change the collection plate between each elution to prevent unnecessary dilution of the target protein.
Vacuum procedure for high-throughput screening
If problems with foaming, reproducibility, or bubbles in the collection plate occur using vacuum, the centrifugation procedure should be considered. The distance between the filter plate and the collection plate is critical; reduce the distance to 5 mm if necessary.
Preparing the 96-well filter plate
- Peel off the bottom seal from the 96-well filter plate. Be sure to hold the filter plate over a sink to accommodate any leakage of storage solution when removing the bottom seal.
- Hold the filter plate upside down and gently shake it to dislodge any medium adhering to the top seal. Return the filter plate to an upright position.
- Place the filter plate against the bench surface and peel off the top seal.
- Position the filter plate on top of a collection plate.
Note: Remember to change or empty the collection plate as necessary during the following steps. - Set the vacuum to -0.15 bar. Place the 96-well plate and collection plate on the vacuum manifold to remove the ethanol storage solution from the medium.
- Add 500 µl of deionized water to each well. Apply vacuum to drain the water from the wells.
- Add 500 µl of binding buffer to each well to equilibrate the medium. Remove the solution as in step 5. Repeat once. The filter plate is now ready for use.
Blank run: Reducing agents may be used in sample and buffers. In such a case, run a blank run by applying 500 µl of elution buffer/well before step 7. No reducing agent should be used in buffer during blank runs. Reequilibrate with binding buffer that includes reducing agent before sample application. Do not leave His MultiTrap™ plates with buffers that include reducing agents when not in use.
Vacuum procedure
Do not apply a pressure in excess of -0.5 bar during vacuum operation.
If a robotic system is used for purification, the vacuum must be adjusted according to methods applicable to the system.
- Apply unclarified or clarified lysate (maximum 600 µl per well) to the wells of the filter plate and incubate for 3 min.
Note: If the yield of protein is too low, increase the incubation time and/or gently agitate the filter plate. - Remove the flowthrough by applying a vacuum of -0.15 bar until all the wells are empty. Slowly increase the vacuum to -0.30 bar and turn off the vacuum after approximately 5 sec. Discard the flowthrough.
Increasing the vacuum too quickly can result in foaming under the filter plate and subsequent cross-contamination of samples.
- Add 500 µl of binding buffer per well to wash out any unbound sample. Apply a vacuum of -0.15 bar as in step 2. Repeat once or until all unbound sample is removed.
Note: Removal of unbound material can be monitored as A280. For high purity, A280 should be < 0.1. - Add 200 µl of elution buffer per well and mix for 1 min.
Note: The volume of elution buffer can be varied (50 to 200 µl per well), depending on the concentration of target protein required. Volumes in the collection plate that are too small may give inaccurate absorbance values. - Change the collection plate and apply a vacuum of -0.15 bar to collect the eluted protein. Repeat twice or until all the target protein has been eluted.
Note: The yield can usually be monitored for each fraction as A280 reading. If uncertain of required volume, change the collection plate between each elution to prevent unnecessary dilution of the target protein.
Application example
Determining solubility effects of detergents in buffers during purification of membrane proteins using His MultiTrap™ FF
The 96-well filter plate format of His MultiTrap™ FF and His MultiTrap™ HP allows high-throughput screening and purification of histidine-tagged proteins. In this example, His MultiTrap™ FF was used to screen eight detergents for their effect on the solubility of six histidine-tagged membrane proteins. Results from purification screening of two proteins, GlpG protein (EM29) and cation transporter (EM43), are shown in a dot blot and SDS-PAGE in Figure 3.7. The results show that conditions to find the most appropriate detergent for the membrane proteins in the study can be readily optimized, with high reproducibility, using MultiTrap™ 96-well filter plate.

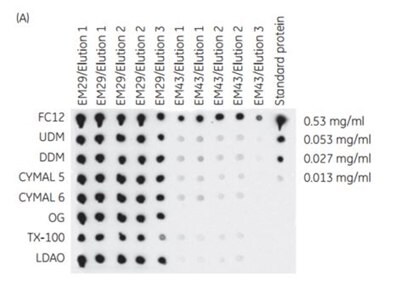
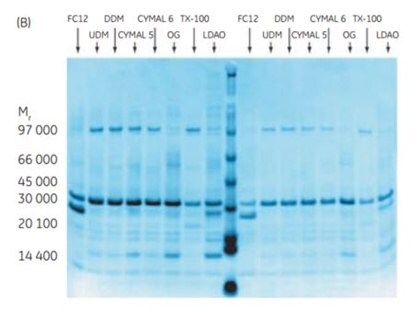
Figure 3.7(A) Dot blot of membrane proteins EM29 and EM43 purified on His MultiTrap™ FF in the presence of different detergents. Repeats of eluates 1 and 2 shown in the dot blot are two independent extractions and purifications. (B) SDS-PAGE (Coomassie staining) of EM29 purifications (elutions 1 and 2 in the blot) with eight different detergents on His MultiTrap™ FF. Data kindly provided by S. Eshagi, V. Lieu, P. Nordlund, Dept. of Medical Biochemistry and Biophysics, Karolinska Institute, Stockholm, Sweden.
To continue reading please sign in or create an account.
Don't Have An Account?