Purifcation or Removal of Serine Proteases, e.g. Thrombin and Trypsin, and Zymogens
Sample extraction procedures often release proteases into solution, requiring the addition of protease inhibitors to prevent unwanted proteolysis. An alternative to the addition of inhibitors is to use a group specific affinity medium to remove the proteases from the sample. The same procedure can be used to either specifically remove these proteases or purify them.
The synthetic inhibitor para-aminobenzamidine is used as the affinity ligand for trypsin, trypsin-like serine proteases and zymogens. Benzamidine Sepharose 4 Fast Flow (high sub) is frequently used to remove molecules from cell culture supernatant, bacterial lysate or serum. During the production of recombinant proteins, tags such as GST are often used to facilitate purification and detection. Enzyme specific recognition sites are included in the recombinant protein to allow the removal of the tag by enzymatic cleavage when required. Thrombin is commonly used for enzymatic cleavage, and must often be removed from the recombinant product. HiTrap® Benzamidine FF (high sub) provides a simple, ready to use solution for this process. Figure 30 shows the partial structure of Benzamidine Sepharose 4 Fast Flow (high sub) and Table 4 gives examples of different serine proteases.
Figure 30.Partial structure of Benzamidine Sepharose 4 Fast Flow (high sub).
Purifcation Options
Purification Examples
Figure 31. shows an example of the removal of trypsin-like proteases from human plasma to prevent proteolysis of the plasma components, using a low pH elution. The activity test demonstrated that almost all trypsin-like protease activity is removed from the sample and bound to the column.
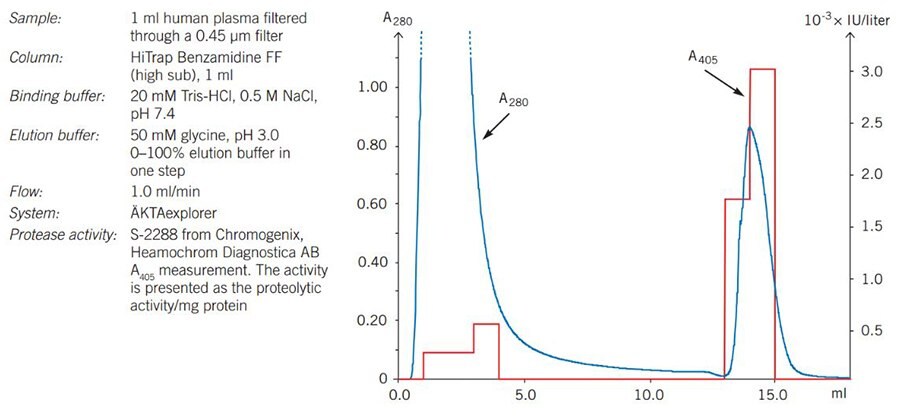
Figure 31.Removal of trypin-like serine proteases from human plasma using HiTrap® Benzamidine FF (high sub), 1 mL.
Figure 32. shows the effectiveness of using a GSTrap FF column with a HiTrap® Benzamidine FF (high sub) for purification of a GST fusion protein, followed by cleavage of the GST tag via the thrombin cleavage site and subsequent removal of the thrombin enzyme. The GST fusion protein binds to the GSTrap FF column as other proteins wash through the column. Thrombin is applied to the column and incubated for 2 hours.
A HiTrap® Benzamidine FF (high sub) column, pre-equilibrated in binding buffer, is attached after the GSTrap FF column and both columns are washed in binding buffer followed by a high salt buffer. The cleaved protein and thrombin wash through from the GSTrap FF column, thrombin binds to the HiTrap® Benzamidine FF (high sub) column, and the eluted fractions contain pure cleaved protein.
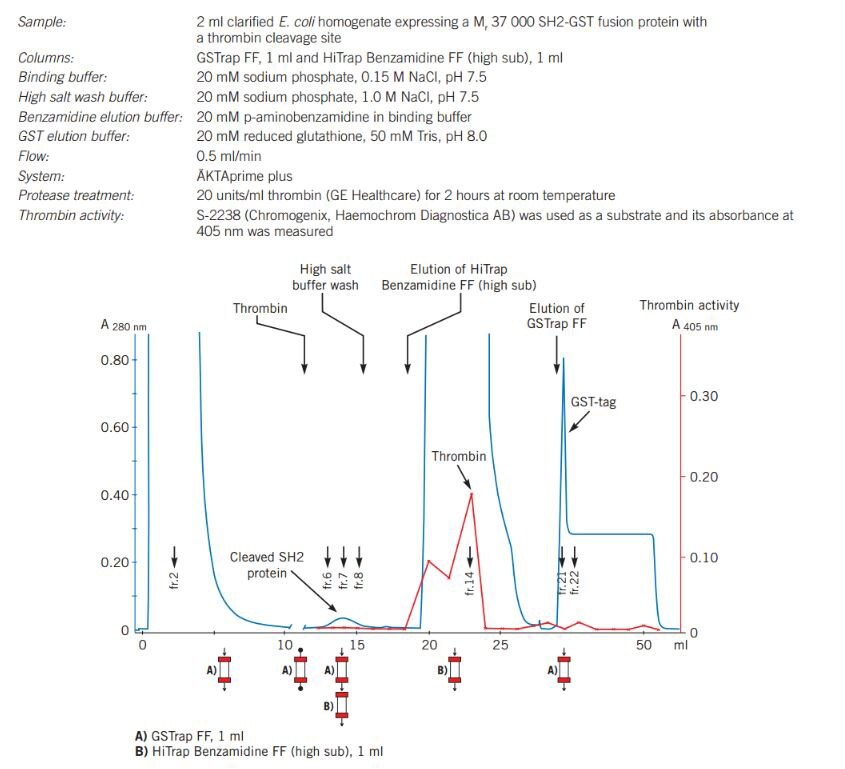
Figure 32.On-column cleavage of a GST fusion protein and removal of thrombin after on-column cleavage, using GSTrap FF and HiTrap® Benzamidine FF (high sub).
Performing a Separation
Binding buffer: 0.05 M Tris-HCl, 0.5 M NaCl, pH 7.4
Elution buffer alternatives:
- pH elution: 0.05 M glycine-HCl, pH 3.0 or 10 mM HCl, 0.05 M NaCl, pH 2.0
- competitive elution: 20 mM p-aminobenzamidine in binding buffer
- denaturing eluents: 8 M urea or 6 M guanidine hydrochloride
- Equilibrate the column with 5 column volumes of binding buffer.
- Apply the sample.
- Wash with 5–10 column volumes of binding buffer or until no material appears in the eluent (monitored by UV absorption at A280 nm).
- Elute with 5–10 column volumes of elution buffer. Collect fractions in neutralization buffer if low pH elution is used*. The purified fractions can be buffer exchanged using desalting columns (see page 133, Buffer exchange and desalting).
* Since elution conditions are quite harsh, collect fractions into neutralization buffer (60 µL – 200 µL 1 M Tris-HCl, pH 9.0 per mL fraction), so that the fnal pH of the fractions will be approximately neutral.
Since Benzamidine Sepharose 4 Fast Flow (high sub) has some ionic binding characteristics, the use of 0.5 M NaCl and pH elution between 7.4–8.0 is recommended. If lower salt concentrations are used, include a high salt wash step after sample application and before elution.
The elution buffer used for competitive elution has a high absorbance at 280 nm. The eluted protein must be detected by other methods, such as an activity assay, total protein or SDS-PAGE analysis. The advantage with competitive elution is that the pH is kept constant throughout the purification.
Cleaning
Wash with 3–5 column volumes of 0.1 M Tris-HCl, 0.5 M NaCl, pH 8.5 followed with 3–5 column volumes of 0.1 M sodium acetate, 0.5 M NaCl, pH 4.5 and re-equilibrate immediately with 3–5 column volumes of binding buffer.
Remove severe contamination by washing with non-ionic detergent such as 0.1% Triton X-100 at +37 °C for 1 minute.
Media Characteristics
Chemical Stability
All commonly used aqueous buffers.
Storage
Wash media and columns with 20% ethanol in 0.05 M sodium acetate, pH 4.0 (use approximately 5 column volumes for packed media) and store at +4 to +8 °C.
To continue reading please sign in or create an account.
Don't Have An Account?