おすすめの製品
詳細
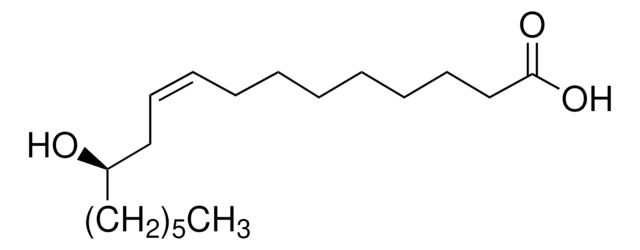
15-Hydroxypentadecanoic acid is an ω-hydroxy acid. One of the method reported for its synthesis is from 1,12-dodecanolide. It is reported to be one of the bioactive component in Tagetes erecta L. leaf and flower extract.
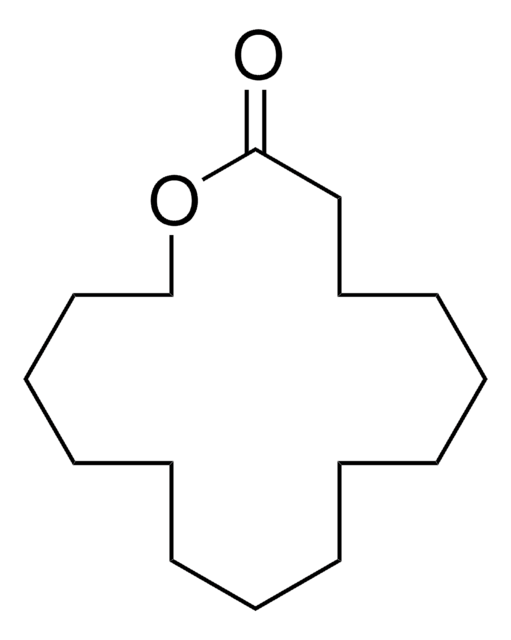
15-Hydroxypentadecanoic acid undergoes lactonization reaction catalyzed by Mucor javanicus L46 and Mucor miehei to afford macrocyclic mono- and oligolactone derivatives. Its lipase-catalyzed synthesis from 15-tetracosenoic acid in Malania Olcifera Chum oil has been proposed. It also participates in the biosynthesis of pentadecanolide.
アプリケーション
15-Hydroxypentadecanoic acid is suitable reagent used in the following studies:
- As an internal standard in the quantification of formation of 11-hydroxylauric acid by gas chromatography.
- In the synthesis of [16-14C]16DCA (DCA= dicarboxylic acid) by one-carbon elongation procedure at C15.
- As an internal standard for the normalization of intensities in the mass spectra of plant cutin polymer.
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
Eyeshields, Gloves, type N95 (US)
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
392979-5G:
392979-VAR:
392979-BULK:
392979-1G:
この製品を見ている人はこちらもチェック
Sacha Ferdinandusse et al.
Journal of lipid research, 45(6), 1104-1111 (2004-04-03)
Dicarboxylic acids (DCAs) are omega-oxidation products of monocarboxylic acids. After activation by a dicarboxylyl-CoA synthetase, the dicarboxylyl-CoA esters are shortened via beta-oxidation. Although it has been studied extensively where this beta-oxidation process takes place, the intracellular site of DCA oxidation
Jamal Mustafa et al.
Lipids, 39(2), 167-172 (2004-05-12)
Derivatives of podophyllotoxin were prepared by coupling 10 FA with the C4-alpha-hydroxy function of podophyllotoxin. The coupling reactions between FA and podophyllotoxin were carried out by dicyclohexylcarbodiimide in the presence of a catalytic amount of dimethylaminopyridine to produce quantitative yields
Enzymatic lactonization of 15-hydroxypentadecanoic and 16-hydroxyhexadecanoic acids to macrocyclic lactones.
Antczak U, et al.
Enzyme and Microbial Technology, 13(7), 589-593 (1991)
Lipase catalyzed synthesis of pentadecanolide from 15-hydroxypentadecanoic acid.
Pan XB, et al.
Chinese Journal of Applied Chemistry / Ying Yong Hua Xue, 21(8), 850-852 (2004)
Dušan Veličković et al.
The Plant journal : for cell and molecular biology, 80(5), 926-935 (2014-10-04)
The cutin polymers of different fruit cuticles (tomato, apple, nectarine) were examined using matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI MSI) after in situ release of the lipid monomers by alkaline hydrolysis. The mass spectra were acquired from each coordinate
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)