おすすめの製品
アッセイ
97%
mp
183-184 °C (lit.)
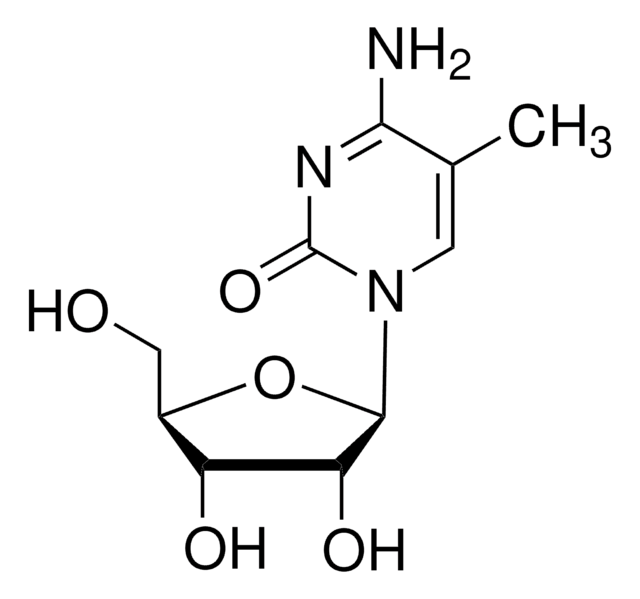
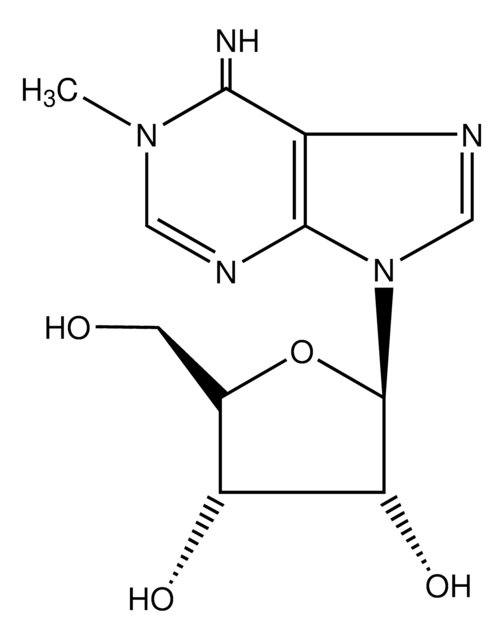
SMILES記法
CC1=CN([C@@H]2O[C@H](CO)[C@@H](O)[C@H]2O)C(=O)NC1=O
InChI
1S/C10H14N2O6/c1-4-2-12(10(17)11-8(4)16)9-7(15)6(14)5(3-13)18-9/h2,5-7,9,13-15H,3H2,1H3,(H,11,16,17)/t5-,6-,7-,9-/m1/s1
InChI Key
DWRXFEITVBNRMK-JXOAFFINSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
Eyeshields, Gloves, type N95 (US)
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
535893-BULK:
535893-25G:
535893-100G:
535893-VAR:
この製品を見ている人はこちらもチェック
Pseudouridine and ribothymidine formation in the tRNA-like domain of turnip yellow mosaic virus RNA.
H F Becker et al.
Nucleic acids research, 26(17), 3991-3997 (1998-08-15)
The last 82 nucleotides of the 6.3 kb genomic RNA of plant turnip yellow mosaic virus (TYMV), the so-called 'tRNA-like' domain, presents functional, structural and primary sequence homologies with canonical tRNAs. In particular, one of the stem-loops resembles the TPsi(pseudouridine)-branch
Marino J E Resendiz et al.
Journal of the American Chemical Society, 134(30), 12478-12481 (2012-07-26)
Photolabile nucleotides that disrupt nucleic acid structure are useful mechanistic probes and can be used as tools for regulating biochemical processes. Previous probes can be limited by the need to incorporate multiple modified nucleotides into oligonucleotides and in kinetic studies
Clive Persaud et al.
Biochemical and biophysical research communications, 392(2), 223-227 (2010-01-14)
Ribosomal RNAs (rRNAs) from all kingdoms contain a variety of post-transcriptional modifications and these are typically clustered in the functional centers of the ribosome. The functions of two bases in the 23S rRNA of Escherichia coli that are post-transcriptionally modified
H An et al.
The Journal of organic chemistry, 66(8), 2789-2801 (2001-04-17)
Novel 5'-O-DMT- and MMT-protected 3'-C-methylene-modified thymidine, 5-methyluridine, and 5-methylcytidine H-phosphonates 1-7 with O-methyl, fluoro, hydrogen, and O-(2-methoxyethyl) substituents at the 2'-position have been synthesized by a new effective strategy from the corresponding key intermediates 3'-C-iodomethyl nucleosides and intermediate BTSP, prepared
S Wang et al.
Biochemistry, 34(12), 4125-4132 (1995-03-28)
Recent studies have shown that there can be large differences in the stability of double and triple helical nucleic acid complexes, depending on whether RNA or DNA strands are involved. These differences have been attributed to structural differences in the
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)