おすすめの製品
詳細
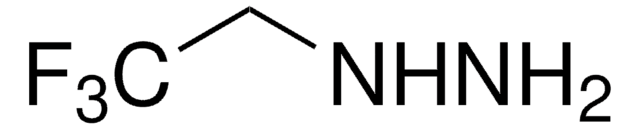
2-Hydroxyethylhydrazine reacts with β-diketones having strong electron-withdrawing substituents to yield 5-hydroxy-4,5-dihydropyrazoles.
その他情報
Sales restrictions may apply
シグナルワード
Danger
危険有害性の分類
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Carc. 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
ターゲットの組織
Respiratory system
保管分類コード
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
引火点(°F)
170.6 °F - closed cup
引火点(℃)
77 °C - closed cup
個人用保護具 (PPE)
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
毒物及び劇物取締法
劇物
消防法
第4類:引火性液体
第三石油類
危険等級III
水溶性液体
労働安全衛生法名称等を表示すべき危険物及び有害物
名称等を表示すべき危険物及び有害物
労働安全衛生法名称等を通知すべき危険物及び有害物
名称等を通知すべき危険物及び有害物
Jan Code
54340-BULK:
54340-500ML:4548173242736
54340-100ML:4548173242729
54340-VAR:
Reaction of 2-hydroxyethylhydrazine with a trifluoromethyl-?-diketone: Study and structural characterization of a new 5-hydroxy-5-trifluoromethyl-4, 5-dihydropyrazole intermediate.
Montoya V, et al.
Journal of Fluorine Chemistry, 128(9), 1007-1011 (2007)
Smaail Radi et al.
Molecules (Basel, Switzerland), 21(8), doi:10-doi:10 (2016-08-17)
A pyridylpyrazole bearing a hydroxyethyl substituent group has been synthesized by condensation of (Z)-4-hydroxy-4-(pyridin-2-yl)but-3-en-2-one with 2-hydroxyethylhydrazine. The compound was well characterized and its structure confirmed by single crystal X-ray diffraction. Density functional calculations have been performed using DFT method with
A M Timperio et al.
Xenobiotica; the fate of foreign compounds in biological systems, 33(2), 153-167 (2003-03-08)
1 Furazolidone, a drug widely used in human and veterinary medicine, exhibits inhibition of monoamine oxidase activity, as observed in the tissues of a number of different animal species, including man. The aim of the current study was to determine
R D Slocum et al.
Planta, 183(3), 443-450 (1991-02-01)
An electron-microscopic cytochemical method was used to localize diamine oxidase (DAO) in pea and polyamine oxidase (PAO) in maize (Zea mays L.). The method, based on the precipitation of amine-oxidase-generated H2O2 by CeCl3, was shown to be specific for DAO
Gregory M Sandala et al.
Journal of the American Chemical Society, 127(24), 8856-8864 (2005-06-16)
Ab initio molecular orbital calculations have been used to study the mechanism of suicide inactivation of ethanolamine ammonia-lyase induced by three different substrate analogues. Analysis of the normal catalytic mechanism with 2-aminoethanol (ethanolamine) as substrate predicts that both the hydrogen-abstraction
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)