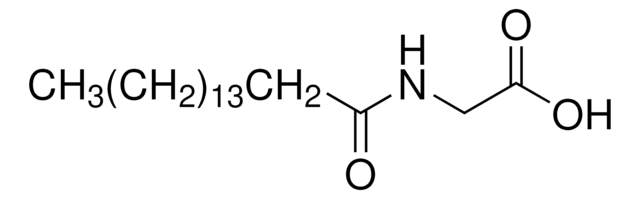
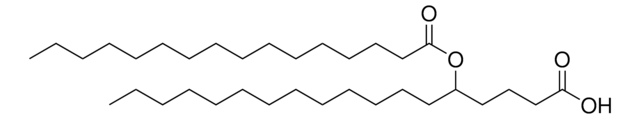
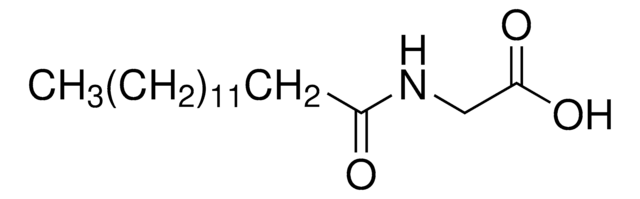
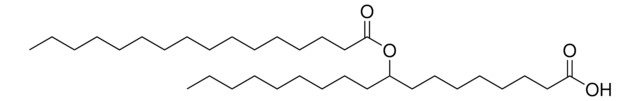
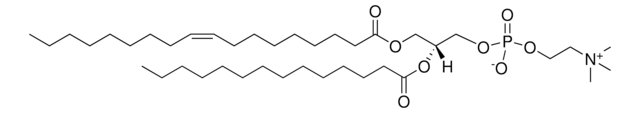
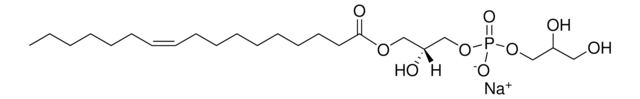
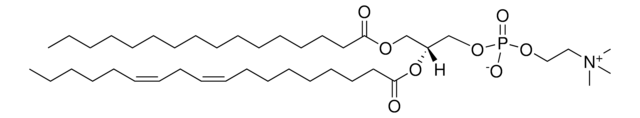
おすすめの製品
フォーム
powder
包装
pkg of 1 × 10 mg (870817P-10mg)
メーカー/製品名
Avanti Research™ - A Croda Brand
脂質タイプ
neutral glycerides
neutral lipids
輸送温度
dry ice
保管温度
−20°C
SMILES記法
O=C(CCCCCCCCCCCCCCC)NCC(O)=O
InChI
1S/C18H35NO3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-17(20)19-16-18(21)22/h2-16H2,1H3,(H,19,20)(H,21,22)
InChI Key
KVTFEOAKFFQCCX-UHFFFAOYSA-N
詳細
N-palmitoylglycine belongs to the family of acyl amides, which are endogenous lipids with a potential to modulate pain and inflammation. Structurally, N-palmitoylglycine (PalGly) comprises 16-carbon saturated fatty acid attached to glycine through amide linkage. It is a structural analog of phospholipid-derived N-acyl ethanolamine. N-palmitoylglycine, a saturated lipid is endogenously produced at high levels in rat skin and spinal cord.
アプリケーション
N-palmitoylglycine has been used as an intact polar lipid (IPL) standard for determination of δ 15N values of nitrogen containing headgroups of IPLs using gas chromatography/combustion/isotope-ratio mass spectrometry.
生物化学的/生理学的作用
N-palmitoylglycine stimulates calcium influx and nitric oxide (NO) production through calcium-sensitive nitric-oxide synthase in sensory neurons. It acts as a lipid activator of G-protein-coupled receptor GPR132.
包装
5 mL Amber Glass Screw Cap Vial (870817P-10mg)
法的情報
Avanti Research is a trademark of Avanti Polar Lipids, LLC
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
870817P-BULK:
870817P-VAR:
870817P-10MG:
最新バージョンのいずれかを選択してください:
Cyrine Ezzili et al.
Bioorganic & medicinal chemistry letters, 20(20), 5959-5968 (2010-09-08)
Key studies leading to the discovery and definition of the role of endogenous fatty acid amide signaling molecules are summarized.
Neta Rimmerman et al.
Molecular pharmacology, 74(1), 213-224 (2008-04-22)
N-arachidonoyl glycine is an endogenous arachidonoyl amide that activates the orphan G protein-coupled receptor (GPCR) GPR18 in a pertussis toxin (PTX)-sensitive manner and produces antinociceptive and antiinflammatory effects. It is produced by direct conjugation of arachidonic acid to glycine and
Elisabeth Svensson et al.
Rapid communications in mass spectrometry : RCM, 29(23), 2263-2271 (2015-11-03)
Compound-specific isotope analysis (CSIA) of nitrogen in amino acids has proven a valuable tool in many fields (e.g. ecology). Several intact polar lipids (IPLs) also contain nitrogen, and their nitrogen isotope ratios have the potential to elucidate food-web interactions or
Shalini Chaturvedi et al.
Prostaglandins & other lipid mediators, 81(3-4), 136-149 (2006-11-07)
Oleamide (cis-9-octadecenamide) is a member of an emerging class of lipid-signaling molecules, the primary fatty acid amides. A growing body of evidence indicates that oleamide mediates fundamental neurochemical processes including sleep, thermoregulation, and nociception. Nevertheless, the mechanism for oleamide biosynthesis
Heather B Bradshaw et al.
Vitamins and hormones, 81, 191-205 (2009-08-04)
Discovery of the endogenous cannabinoid and N-acyl amide, anandamide (N-arachidonoyl ethanolamine), paved the way for lipidomics discoveries in the growing family of N-acyl amides. Lipidomics is a field that is broadening our view of the molecular world to include a
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)