おすすめの製品
フォーム
powder
包装
pkg of 1 × 5 mg (999995P-5mg)
メーカー/製品名
Avanti Research™ - A Croda Brand 999995P
脂質タイプ
cardiolipins
phospholipids
輸送温度
dry ice
保管温度
−20°C
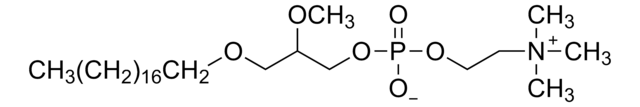
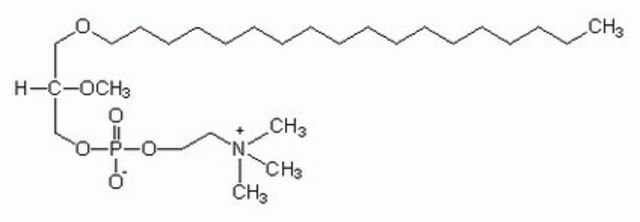
SMILES記法
[O-]P(OCC[N+](C)(C)C)(OC[C@]([H])(OC)COCCCCCCCCCCCCCCCCCC)=O
InChI
1S/C10H24NO5P/c1-6-10(14-5)9-16-17(12,13)15-8-7-11(2,3)4/h10H,6-9H2,1-5H3/t10-/m0/s1
InChI Key
GVMCXWJIRSIWFJ-JTQLQIEISA-N
関連するカテゴリー
アプリケーション
Edelfosine or 1-O-octadecyl-2-O-methyl-sn-glycero-3-phosphocholine might be used:
- as a non-hydrolysable LysoPC (phospholipid) analog for analyzing its ability to block sexual commitment in Plasmodium falciparum
- in multilamellar vesicle preparation, to study its effect on model membranes
- in the selection and screening of mutagenized cells, having the ability to inhibit the transport of alkylphosphocholine drugs across the plasma membrane
生物化学的/生理学的作用
Edelfosine acts as a precursor for alkyl-lysophospholipids. It possesses apoptotic action against several cancer cells such as prostate, leukemia, brain and lung tumors. It is associated with cellular transport system, signaling transducing systems, cytokine synthesis and lipid metabolism. Edelfosine is known to control intracellular calcium levels. It is not mutagenic and its anti-tumor action requires its incorporation into the cell. Edelfosine is also found to block the replication of human immunodeficiency virus type I (HIV-I).
包装
5 mL Clear Glass Sealed Ampule (999995P-5mg)
法的情報
Avanti Research is a trademark of Avanti Polar Lipids, LLC
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
999995P-10MG:
999995P-VAR:
999995P-BULK:
999995P-5MG:
999995P-50MG:
最新バージョンのいずれかを選択してください:
Alessio Ausili et al.
The journal of physical chemistry. B, 112(37), 11643-11654 (2008-08-21)
The effect of edelfosine (1- O-octadecyl-2- O-methyl-rac-glycero-3-phosphocholine or ET-18-OCH3) on model membranes containing 1-palmitoyl-2-oleoyl- sn-glycero-3-phosphocholine/sphingomyelin/cholesterol (POPC/SM/cholesterol) was studied by several physical techniques. The sample POPC/SM (1:1 molar ratio) showed a broad phase transition as seen by DSC, X-ray diffraction, and
Pamela K Hanson et al.
The Journal of biological chemistry, 278(38), 36041-36050 (2003-07-05)
The alkylphosphocholine class of drugs, including edelfosine and miltefosine, has recently shown promise in the treatment of protozoal and fungal diseases, most notably, leishmaniasis. One of the major barriers to successful treatment of these infections is the development of drug
Bruno M Castro et al.
The journal of physical chemistry. B, 117(26), 7929-7940 (2013-06-07)
Edelfosine (1-O-octadecyl-2-O-methyl-sn-glycero-phosphocholine) and miltefosine (hexadecylphosphocholine) are synthetic alkylphospholipids (ALPs) that are reported to selectively accumulate in tumor cell membranes, inducing Fas clustering and activation on lipid rafts, triggering apoptosis. However, the exact mechanism by which these lipids elicit these events
Nicolas M B Brancucci et al.
Cell, 171(7), 1532-1544 (2017-11-14)
Transmission represents a population bottleneck in the Plasmodium life cycle and a key intervention target of ongoing efforts to eradicate malaria. Sexual differentiation is essential for this process, as only sexual parasites, called gametocytes, are infective to the mosquito vector. Gametocyte production
Alessio Ausili et al.
Langmuir : the ACS journal of surfaces and colloids, 34(28), 8333-8346 (2018-06-21)
Edelfosine is an anticancer drug with an asymmetric structure because, being a derivative of glycerol, it possesses two hydrophobic substituents of very different lengths. We showed that edelfosine destabilizes liquid-ordered membranes formed by either 1-palmitoyl-2-oleoyl- sn-glycero-3-phosphocholine, sphingomyelin (SM), and cholesterol
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)