おすすめの製品
品質水準
製品種目
Novabiochem®
形状
beads
反応適合性
reaction type: Fmoc solid-phase peptide synthesis
メーカー/製品名
Novabiochem®
アプリケーション
peptide synthesis
官能基
alcohol
保管温度
2-30°C
詳細
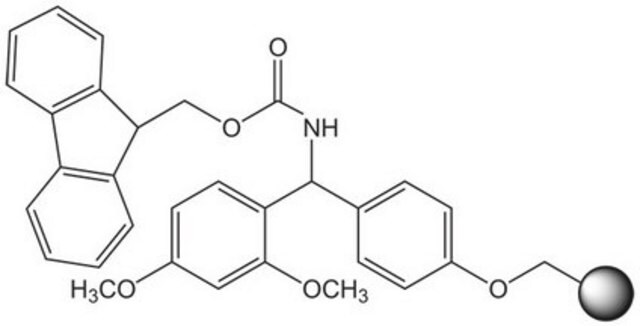
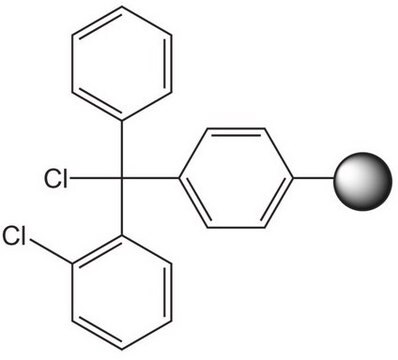
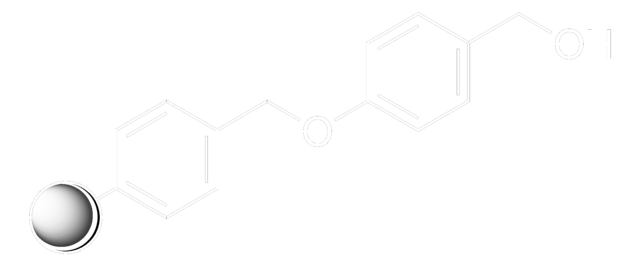
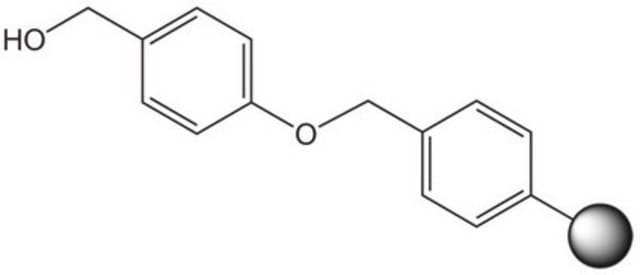
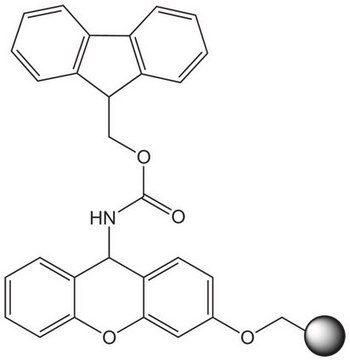
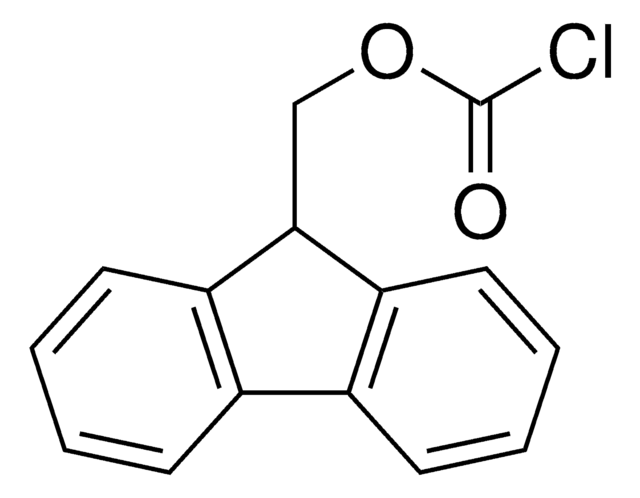
Rink acid resin is a super acid-labile support for the solid phase immobilization of carboxylic acids [1] by Fmoc SPPS. Cleavage can be effected with as little as 10% AcOH in DCM, providing highly acid-sensitive products in high yields and purities. However, care must be taken to prevent product loss during synthetic manipulations [2], owing to the extreme acid sensitivity of this support. Treatment with HCl in THF [3] or Ph2PCl2 [4] has been shown to efficiently convert this resin to the corresponding benzhydryl chloride, to which can be coupled a wide range of functional groups: hydroxylamines [3], alcohols, amines, acids and thiols [4]. Rink acid resin has also been converted to a trifluoroacetate with TFA and used in a similar manner to immobilize amines, thiols, alcohols, and phenols [5]. In a more detailed study, the same authors found 1 M trifluoroacetic anhydride in 2,6-lutidine to give superior results with less degradation of the linker [6, 7]. Rink resin trifluoroacetate has also been used to prepare purines [8, 9].Cleavage of amines and alcohols from this support has been carried out with either 5% TFA in DCM [4] or 20% TFA in DCM [5]; thiols were released with either 5% TFA in DCM [4] or 95% aq. TFA [5].
Associated Protocols and Technical Articles
Protocols for Loading of Peptide Synthesis Resins
Literature references
[1] H. Rink (1987) Tetrahedron Lett., 28, 3787.
[2] H. U. Immer, et al. in ′Peptides, Chemistry, Structure &Biology, Proc. 11th American Peptide Symposium′, J. E. Rivier & G. R. Marshall (Eds), ESCOM, Leiden, 1990, pp. 1054.
[3] S. L. Mellor & W. C. Chan (1997) J. Chem. Soc., Chem. Commun., 2005.
[4] R. S. Garigipati (1997) Tetrahedron Lett., 38, 6807.
[5] W.K.-D. Brill in ′First Conference on Synthetic Organic Chemistry′, www.mdpi.org/ecsoc, 1997.
[6] W.K.-D. Brill (1998) Syn. Lett., 906.
[7] R. A. Tommesi, et al. (1998) Tetrahedron Lett., 39, 5477.
[8] W.K.-D. Brill (2001) Syn. Lett., 1097.
[9] W.K.-D. Brill & C. Riva-Toniolo (2001) Tetrahedron Lett., 42, 65`5.
Associated Protocols and Technical Articles
Protocols for Loading of Peptide Synthesis Resins
Literature references
[1] H. Rink (1987) Tetrahedron Lett., 28, 3787.
[2] H. U. Immer, et al. in ′Peptides, Chemistry, Structure &Biology, Proc. 11th American Peptide Symposium′, J. E. Rivier & G. R. Marshall (Eds), ESCOM, Leiden, 1990, pp. 1054.
[3] S. L. Mellor & W. C. Chan (1997) J. Chem. Soc., Chem. Commun., 2005.
[4] R. S. Garigipati (1997) Tetrahedron Lett., 38, 6807.
[5] W.K.-D. Brill in ′First Conference on Synthetic Organic Chemistry′, www.mdpi.org/ecsoc, 1997.
[6] W.K.-D. Brill (1998) Syn. Lett., 906.
[7] R. A. Tommesi, et al. (1998) Tetrahedron Lett., 39, 5477.
[8] W.K.-D. Brill (2001) Syn. Lett., 1097.
[9] W.K.-D. Brill & C. Riva-Toniolo (2001) Tetrahedron Lett., 42, 65`5.
関連事項
Replaces: 01-64-0012
アナリシスノート
Color (visual): white to yellow to beige
Appearance of substance (visual): beads
Loading (determined from the substition of the Fmoc-NH2 loaded resin): 0.35 - 0.80 mmol/g
Swelling Volume (in DMF): lot specific result
The polymer matrix is copoly (styrene - 1% DVB) 100 -200 mesh
Appearance of substance (visual): beads
Loading (determined from the substition of the Fmoc-NH2 loaded resin): 0.35 - 0.80 mmol/g
Swelling Volume (in DMF): lot specific result
The polymer matrix is copoly (styrene - 1% DVB) 100 -200 mesh
法的情報
Novabiochem is a registered trademark of Merck KGaA, Darmstadt, Germany
保管分類コード
11 - Combustible Solids
WGK
WGK 1
引火点(°F)
Not applicable
引火点(℃)
Not applicable
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
資料
Novabiochem® offers a wide range of linkers and derivatized resins for Fmoc solid-phase peptide synthesis with specialized protocols.
プロトコル
Review various resins like Merrifield, trityl-based, and hydroxymethyl-functionalized for peptide immobilization for diverse applications.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)