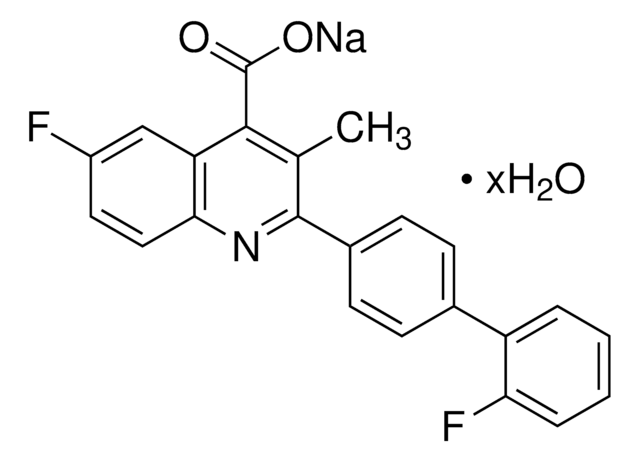
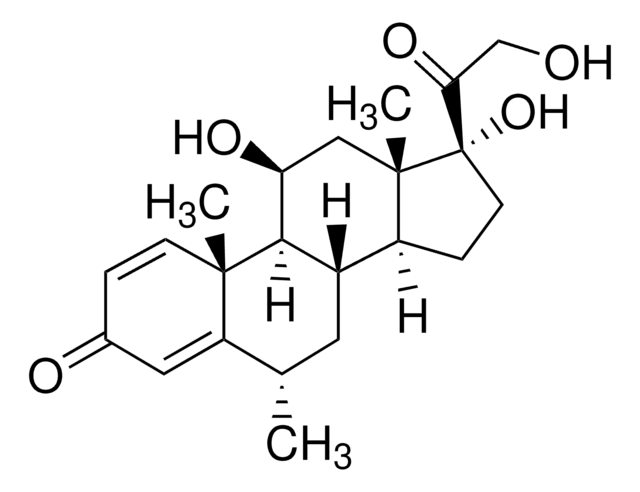
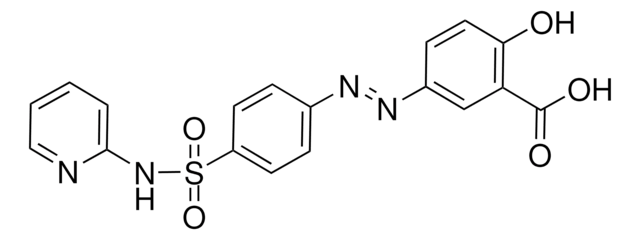
おすすめの製品
グレード
certified reference material
pharmaceutical secondary standard
品質水準
認証
traceable to Ph. Eur. Y0000654
traceable to USP 1356960
APIファミリー
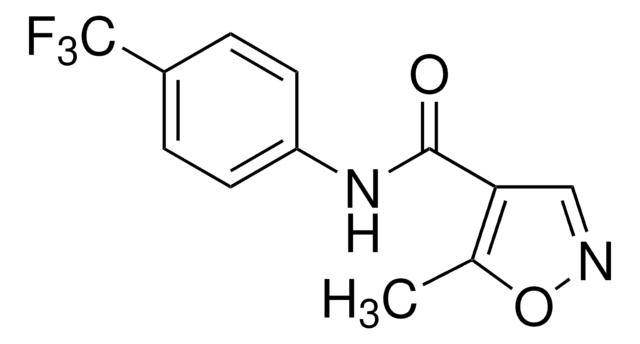
leflunomide
CofA
current certificate can be downloaded
テクニック
HPLC: suitable
gas chromatography (GC): suitable
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
保管温度
2-8°C
SMILES記法
Cc1oncc1C(=O)Nc2ccc(cc2)C(F)(F)F
InChI
1S/C12H9F3N2O2/c1-7-10(6-16-19-7)11(18)17-9-4-2-8(3-5-9)12(13,14)15/h2-6H,1H3,(H,17,18)
InChI Key
VHOGYURTWQBHIL-UHFFFAOYSA-N
遺伝子情報
human ... DHODH(1723)
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
アプリケーション
アナリシスノート
その他情報
脚注
関連製品
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
ターゲットの組織
Respiratory system
保管分類コード
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 1
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
PHR1378-1G:
PHR1378-1G-PW:
この製品を見ている人はこちらもチェック
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)