おすすめの製品
グレード
pharmaceutical primary standard
APIファミリー
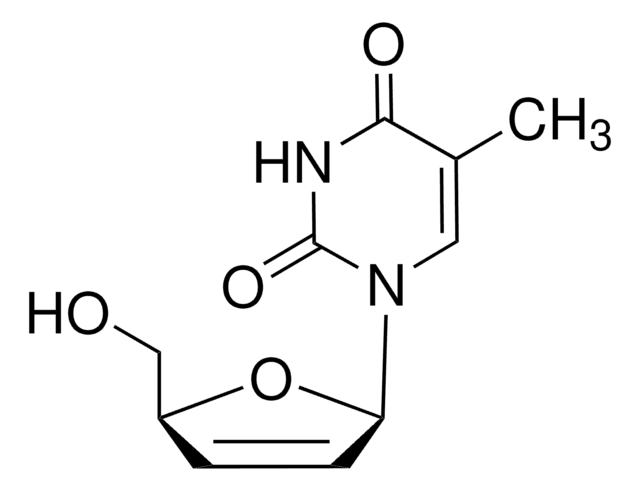
stavudine
メーカー/製品名
EDQM
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
保管温度
2-8°C
SMILES記法
CC1=CN([C@@H]2O[C@H](CO)C=C2)C(=O)NC1=O
InChI
1S/C10H12N2O4/c1-6-4-12(10(15)11-9(6)14)8-3-2-7(5-13)16-8/h2-4,7-8,13H,5H2,1H3,(H,11,14,15)/t7-,8+/m0/s1
InChI Key
XNKLLVCARDGLGL-JGVFFNPUSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
この製品は薬局方標準品です。発行元の薬局方により製造・供給されています。MSDSを含む製品情報などの詳しい情報は、発行元の薬局方のウェブサイトよりご確認ください。
アプリケーション
Stavudine EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包装
この製品は発行元の薬局方による供給としてお届けします。現在の単位量については、EDQM reference substance catalogueをご覧ください。
その他情報
Sales restrictions may apply.
関連製品
製品番号
詳細
価格
保管分類コード
11 - Combustible Solids
WGK
WGK 2
引火点(°F)
Not applicable
引火点(℃)
Not applicable
最新バージョンのいずれかを選択してください:
この製品を見ている人はこちらもチェック
M Hurst et al.
Drugs, 58(5), 919-949 (1999-12-14)
Stavudine is a thymidine nucleoside analogue which is phosphorylated intracellularly to an active metabolite, stavudine 5'-triphosphate. This metabolite inhibits HIV replication, either by competing with thymidine 5'-triphosphate for incorporation into viral DNA by reverse transcriptase or by causing premature termination
Susan M Cheer et al.
Drugs, 62(18), 2667-2674 (2002-12-06)
Stavudine administered once daily is a nucleoside analogue reverse transcriptase inhibitor. The efficacy (reduction in viral load and increase in CD4+ lymphocyte counts from baseline) of stavudine once daily-containing triple therapy was similar to that of stavudine immediate release (IR)-containing
S A Riddler et al.
Antiviral research, 27(3), 189-203 (1995-06-01)
Stavudine, 2',3'-didehydro-3'-deoxythymidine (D4T), is a potent inhibitor of HIV-1 reverse transcriptase in vitro. In clinical studies, stavudine has excellent oral bioavailability in excess of 80%. The dose-limiting toxicity is peripheral neuropathy, which occurred in 15% of stavudine versus 6% of
Alain Makinson et al.
Expert opinion on drug safety, 7(3), 283-293 (2008-05-09)
Western randomized trials and prospective cohorts in resource-limited settings have proven virological success with stavudine-based highly active antiretroviral therapy. However, stavudine is no longer recommended in first-line treatments in these two settings due to its intrinsic toxicities and side effects.
N Clumeck
Antiviral therapy, 3 Suppl 4, 39-43 (2000-03-21)
Current guidelines for treatment of human immunodeficiency virus (HIV) disease favour the use of triple-drug combinations consisting of two nucleoside analogue reverse transcriptase inhibitors (NRTIs) plus a protease inhibitor to achieve maximum suppression of HIV replication. There is considerable evidence
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)