おすすめの製品
グレード
pharmaceutical primary standard
APIファミリー
trospium
メーカー/製品名
EDQM
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
保管温度
2-8°C
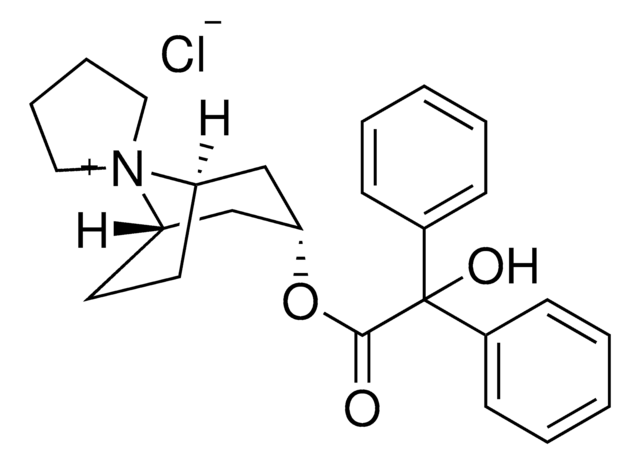
SMILES記法
[Cl-].[N+]21(C3CCC2CC(C3)OC(=O)C(O)(c5ccccc5)c4ccccc4)CCCC1
InChI
1S/C25H30NO3.ClH/c27-24(25(28,19-9-3-1-4-10-19)20-11-5-2-6-12-20)29-23-17-21-13-14-22(18-23)26(21)15-7-8-16-26;/h1-6,9-12,21-23,28H,7-8,13-18H2;1H/q+1;/p-1
InChI Key
RVCSYOQWLPPAOA-UHFFFAOYSA-M
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
この製品は薬局方標準品です。発行元の薬局方により製造・供給されています。MSDSを含む製品情報などの詳しい情報は、発行元の薬局方のウェブサイトよりご確認ください。
アプリケーション
Trospium chloride EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包装
この製品は発行元の薬局方による供給としてお届けします。現在の単位量については、EDQM reference substance catalogueをご覧ください。
その他情報
Sales restrictions may apply.
最新バージョンのいずれかを選択してください:
Norman R Zinner et al.
Neurourology and urodynamics, 30(7), 1214-1219 (2011-04-05)
Once-daily extended-release (XR) trospium chloride has been evaluated for the treatment of overactive bladder syndrome (OAB) in two 12-week randomized, double-blind, placebo-controlled studies. This pooled analysis of the 9-month open-label extensions to these studies evaluated the long-term efficacy and tolerability
David A Ginsberg et al.
Neurourology and urodynamics, 30(4), 563-567 (2011-01-27)
Once-daily extended release (XR) trospium chloride, which provides therapeutic trospium plasma concentrations over 24 hours, has demonstrated efficacy in treating overactive bladder (OAB) symptoms as evaluated over a 24-hr period. This analysis examined the effects of trospium XR on diurnal
Anthony G Visco et al.
The New England journal of medicine, 367(19), 1803-1813 (2012-10-06)
Anticholinergic medications and onabotulinumtoxinA are used to treat urgency urinary incontinence, but data directly comparing the two types of therapy are needed. We performed a double-blind, double-placebo-controlled, randomized trial involving women with idiopathic urgency urinary incontinence who had five or
Mark D Harnett et al.
Clinical drug investigation, 33(2), 133-141 (2012-12-04)
Overactive bladder is a common disorder that affects approximately 34 million adults in the United States. Anticholinergic (antimuscarinic) agents are the most widely used pharmacological option for overactive bladder. This study set out to identify and characterize the influence of
Peter K Sand et al.
Drugs & aging, 28(2), 151-160 (2011-02-01)
Overactive bladder syndrome (OAB) is associated with various co-morbidities; treatment of these frequently results in multiple medication use (MMU) and the potential for drug-drug interactions, which may lead to adverse events and altered efficacy. With the aging population, the prevalence
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)