おすすめの製品
由来生物
synthetic
品質水準
アッセイ
≥98% (HPLC)
フォーム
solid
溶解性
ethanol: 50 mg/mL
抗生物質活性スペクトル
viruses
作用機序
enzyme | inhibits
保管温度
−20°C
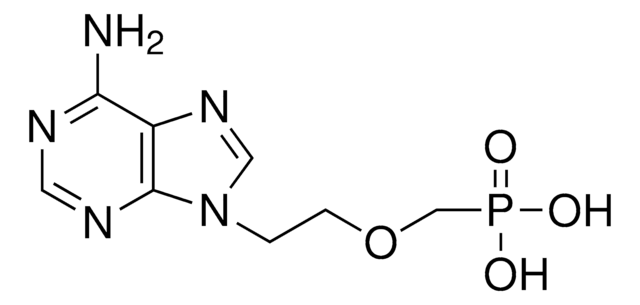
SMILES記法
CC(C)(C)C(=O)OCOP(=O)(COCCn1cnc2c(N)ncnc12)OCOC(=O)C(C)(C)C
InChI
1S/C20H32N5O8P/c1-19(2,3)17(26)30-11-32-34(28,33-12-31-18(27)20(4,5)6)13-29-8-7-25-10-24-14-15(21)22-9-23-16(14)25/h9-10H,7-8,11-13H2,1-6H3,(H2,21,22,23)
InChI Key
WOZSCQDILHKSGG-UHFFFAOYSA-N
生物化学的/生理学的作用
Adefovir is an antiviral acyclic nucleoside phosphonate (ANP) analog that works by blocking reverse transcriptase. It is an inhibitor of duck hepatitis B virus (DHBV) replication that inhibits covalently closed circular DNA (CCC DNA) amplification. Cytotxicity is induced by Human Renal Organic Anion Transporter 1 (hOAT1). Adefovir is an Inhibitor of edema factor (EF)-induced cAMP accumulation and changes in cytokine production in mouse primary macrophages .
その他情報
Keep container tightly closed in a dry and well-ventilated place.
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Acute Tox. 4 Oral
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
dust mask type N95 (US), Eyeshields, Gloves
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
A9730-25MG:
A9730-100MG:
A9730-VAR:
A9730-50MG:
A9730-BULK:
Baek Gyu Jun et al.
PloS one, 13(7), e0201316-e0201316 (2018-07-31)
Convincing data that support routine use of preventive therapy against hepatitis B virus (HBV) reactivation in radiotherapy (RT) for hepatocellular carcinoma (HCC) are lacking. The aim of this study was to investigate the incidence, clinical significance, and risk factors of
A Takeda et al.
Journal of viral hepatitis, 14(2), 75-88 (2007-01-25)
Standard treatments for chronic hepatitis B (CHB) include interferon-alpha (IFN-alpha) and lamivudine (LAM), but these are associated with adverse effects and viral resistance, respectively. The aim of this systematic review and economic evaluation was to assess the clinical effectiveness and
Julien Delmas et al.
Antimicrobial agents and chemotherapy, 46(2), 425-433 (2002-01-18)
The elimination of viral covalently closed circular DNA (CCC DNA) from the nucleus of infected hepatocytes is an obstacle to achieving sustained viral clearance during antiviral therapy of chronic hepatitis B virus (HBV) infection. The aim of our study was
F Stelma et al.
Journal of viral hepatitis, 24(12), 1107-1113 (2017-06-21)
Combining peginterferon-alfa-2a (pegIFN) with a nucleotide analogue can result in higher rates of HBsAg loss than either therapy given alone. Here, we investigated the durability of the response to combination therapy in chronic hepatitis B (CHB) patients after 5 years of
M Danta et al.
International journal of clinical practice, 58(9), 877-886 (2004-11-09)
Adefovir dipivoxil (ADF) is a novel acyclic nucleoside analogue that has recently been approved for the treatment of chronic hepatitis B virus (HBV). Adefovir was initially assessed at higher doses for the treatment of human immunodeficiency virus (HIV) infection. However
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)