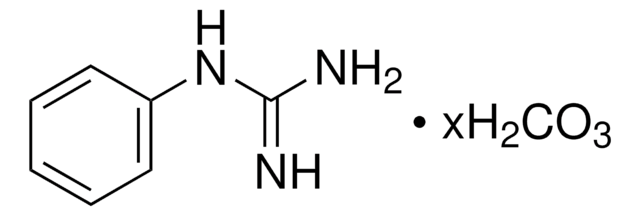
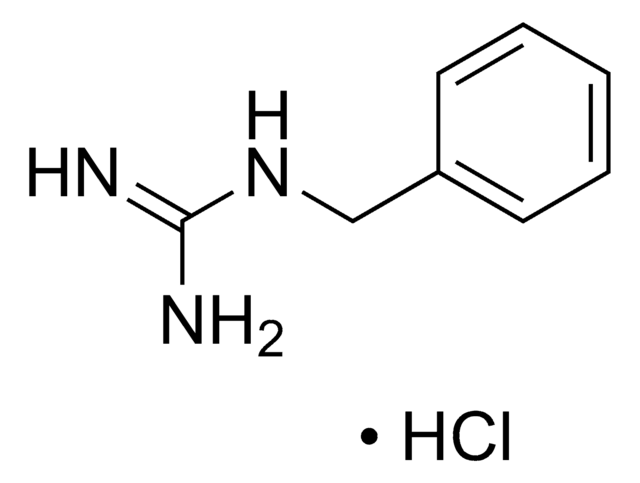
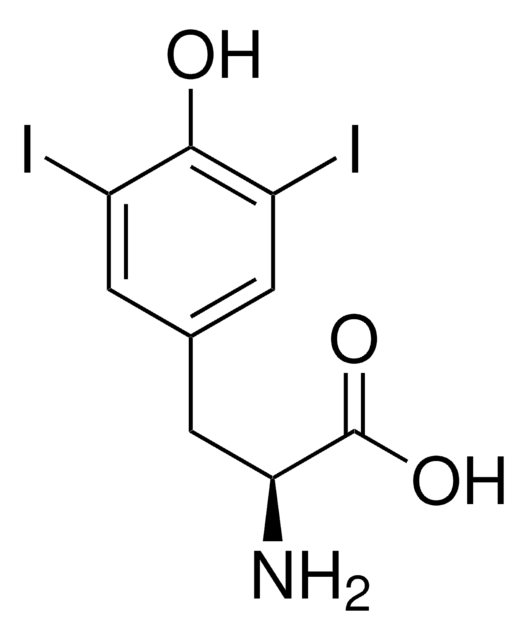
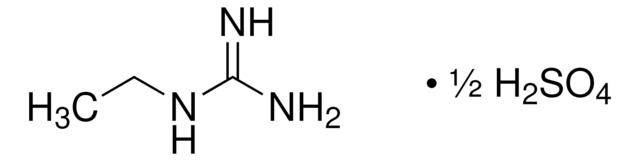
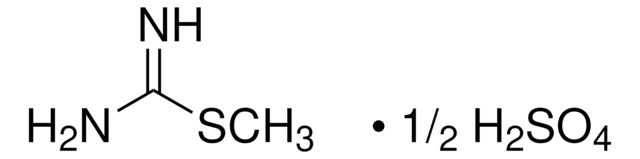おすすめの製品
由来生物
synthetic (organic)
品質水準
アッセイ
≥98% (TLC)
フォーム
powder
溶解性
water: 25 mg/mL, clear, colorless
保管温度
2-8°C
SMILES記法
NC(=N)NO.NC(=N)NO.OS(O)(=O)=O
InChI
1S/2CH5N3O.H2O4S/c2*2-1(3)4-5;1-5(2,3)4/h2*5H,(H4,2,3,4);(H2,1,2,3,4)
InChI Key
MTGDDPZRXSDPFH-UHFFFAOYSA-N
生物化学的/生理学的作用
早期の抗腫瘍薬です。酸化によってNOが遊離され、ペルオキシナイトライトやペルオキシラジカルなど、他の活性酸素種が生成されます。NOと反応して、強力かつ安定した血管拡張剤である一種の付加体を生成します。
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
Eyeshields, Gloves, type N95 (US)
Zhi-guo Zhang et al.
Yao xue xue bao = Acta pharmaceutica Sinica, 39(9), 705-710 (2004-12-21)
To search for novel antiasthmatic agents. Coupling seratrodast (SD), an antiasthmatic drug, with several different types of NO donors including oxatriazoles, N-hydroxyguanidines and furoxans; evaluating the antiasthmatic effects of coupled compounds by determining their inhibitory activity of guinea pig asthma
Daniel Mansuy et al.
Free radical biology & medicine, 37(8), 1105-1121 (2004-09-29)
Nitric oxide (NO) is a key inter- and intracellular molecule involved in the maintenance of vascular tone, neuronal signaling, and host response to infection. The biosynthesis of NO in mammals involves a two-step oxidation of L-arginine (L-Arg) to citrulline and
Patrick Slama et al.
Journal of inorganic biochemistry, 103(3), 455-462 (2009-01-31)
N-Aryl-N'-hydroxyguanidines are compounds that display interesting pharmacological properties but their chemical reactivity remains poorly investigated. Some of these compounds are substrates for the heme-containing enzymes nitric-oxide synthases (NOS) and act as reducing co-substrates for the copper-containing enzyme Dopamine beta-Hydroxylase (DBH)
David Lefèvre-Groboillot et al.
The FEBS journal, 272(12), 3172-3183 (2005-06-16)
The binding of several alkyl- and aryl-guanidines and N-hydroxyguanidines to the oxygenase domain of inducible NO-synthase (iNOS(oxy)) was studied by UV/Vis difference spectroscopy. In a very general manner, monosubstituted guanidines exhibited affinities for iNOS(oxy) that were very close to those
Patrick Slama et al.
Biochemical and biophysical research communications, 316(4), 1081-1087 (2004-03-27)
Conversion of neurotransmitter dopamine into norepinephrine is catalyzed by dopamine beta-hydroxylase (DbH). The reaction requires the presence of both molecular oxygen and a reducing cosubstrate, the assumed physiological cosubstrate being ascorbic acid. We have investigated the ability of a new
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)