おすすめの製品
グレード
pharmaceutical primary standard
APIファミリー
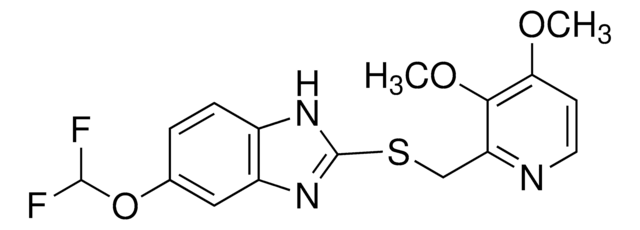
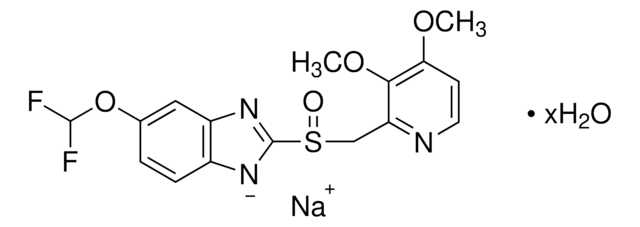
pantoprazole
メーカー/製品名
USP
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
InChI
1S/C16H15F2N3O5S/c1-24-13-5-6-19-12(14(13)25-2)8-27(22,23)16-20-10-4-3-9(26-15(17)18)7-11(10)21-16/h3-7,15H,8H2,1-2H3,(H,20,21)
InChI Key
FCJYMBZQIJDMMM-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
アプリケーション
- Transcriptional profiling for drug repurposing in glioblastoma: This study utilized transcriptional profiling to identify therapeutic targets and repurpose drugs, including pantoprazole-related compounds, for treatment in glioblastoma. This approach underscores the utility of pantoprazole impurities in exploring new therapeutic avenues in pharmaceutical reference material research (Roddy et al., 2023).
- Gastroprotective agent utilization: A drug utilization study in medicine and surgery wards highlighted the significant role of pantoprazole and its related compounds in managing gastrointestinal protection. This research emphasizes the importance of pantoprazole as a proton pump inhibitor and its impurities in ensuring the efficacy and safety of gastroprotective therapies (Koyani et al., 2023).
- Synthesis and analysis of pantoprazole sodium sesquihydrate-related compound E: This study explored the synthesis and mechanism of formation of a specific pantoprazole-related compound, offering insights into the chemical stability and quality control of pantoprazole as a pharmaceutical ingredient. Such research is crucial for enhancing analytical methods in pharmaceutical research and ensuring drug safety and efficacy (Yari et al., 2019).
アナリシスノート
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
その他情報
Sales restrictions may apply.
関連製品
製品番号
詳細
価格
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
E Doyle et al.
Journal of chromatography, 527(1), 67-77 (1990-04-27)
This paper describes two fully automated assays. One for zaprinast, a cGMP specific phosphodiesterase inhibitor, which uses the Gilson-Advanced Automated Sample Processor combination, and the other for an H+/K+ ATPase inhibitor and its sulphone metabolite, which uses direct injection. Both
Dafang Zhong et al.
The Journal of pharmacy and pharmacology, 57(3), 341-349 (2005-04-06)
We have investigated the metabolism of pantoprazole and have provided an explanation for the formation mechanism of its metabolites. Metabolites found in the urine of rats after oral administration of pantoprazole sodium (25 mg kg(-1)) were analysed by liquid chromatography/ion
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)