모든 사진(1)
About This Item
Linear Formula:
CH2=CHPO(OCH2CH3)2
CAS Number:
Molecular Weight:
164.14
Beilstein:
507596
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
97%
refractive index
n20/D 1.429 (lit.)
bp
202 °C (lit.)
density
1.068 g/mL at 25 °C (lit.)
작용기
phosphonate
저장 온도
2-8°C
SMILES string
CCOP(=O)(OCC)C=C
InChI
1S/C6H13O3P/c1-4-8-10(7,6-3)9-5-2/h6H,3-5H2,1-2H3
InChI key
DREPONDJUKIQLX-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Diethyl vinylphosphonate (DEVP) can be used as a precursor for the synthesis of:
It can be also employed as a monomer unit for the preparation of high-molecular-weight polymer, poly(diethyl vinylphosphonate) using lanthanide complexes.{18)
- α, β-unsaturated phosphonates by reacting with arylboronic acids via Pd-catalyzed Mizoroki−Heck reaction.
- 2-(arylamino)ethyl phosphonates by condensing with primary and secondary amines via the aza-Michael addition reaction.
It can be also employed as a monomer unit for the preparation of high-molecular-weight polymer, poly(diethyl vinylphosphonate) using lanthanide complexes.{18)
Diethyl vinylphosphonate has been used in the preparation of diethyl N-alkyl-2-aminoethylphosphonate.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
230.0 °F - closed cup
Flash Point (°C)
110 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Poly (vinylphosphonate) s synthesized by trivalent cyclopentadienyl lanthanide-induced group transfer polymerization
Salzinger S, et al.
Macromolecules, 44(15), 5920-5927 (2011)
Joachim E Klee et al.
Beilstein journal of organic chemistry, 5, 72-72 (2009-01-01)
Novel N-alkyl-N-(phosphonoethyl) substituted mono-, bis- and tris(meth)acrylamides 3 were synthesized by two different three-step reactions and characterized by IR, (1)H NMR and (13)C NMR spectroscopy as well as refractive index and viscosity. The phosphonoethyl substituted (meth)acrylamide monomers show improved hydrolytic
Convenient synthesis of α, β-unsaturated phosphonates via a Mizoroki-Heck reaction of arylboronic acids with diethyl vinylphosphonate
Kabalka GW, et al.
Tetrahedron Letters, 45(24), 4685-4687 (2004)
Synthesis of 2-(arylamino) ethyl phosphonic acids via the aza-Michael addition on diethyl vinylphosphonate
Orm Nadine B, et al.
Tetrahedron, 69(1), 115-121 (2013)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.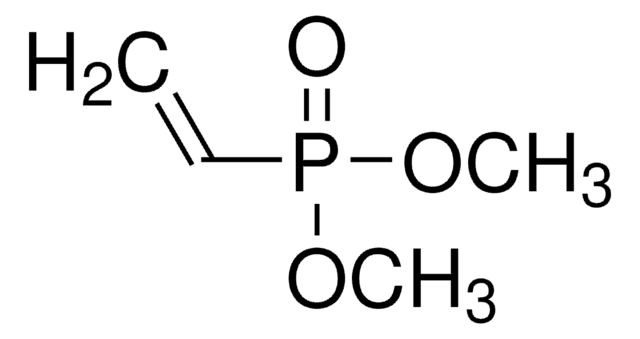


![Bis[2-(methacryloyloxy)ethyl] phosphate](/deepweb/assets/sigmaaldrich/product/structures/128/336/4e7a3e38-338c-423e-95b8-70d9d1f8e121/640/4e7a3e38-338c-423e-95b8-70d9d1f8e121.png)