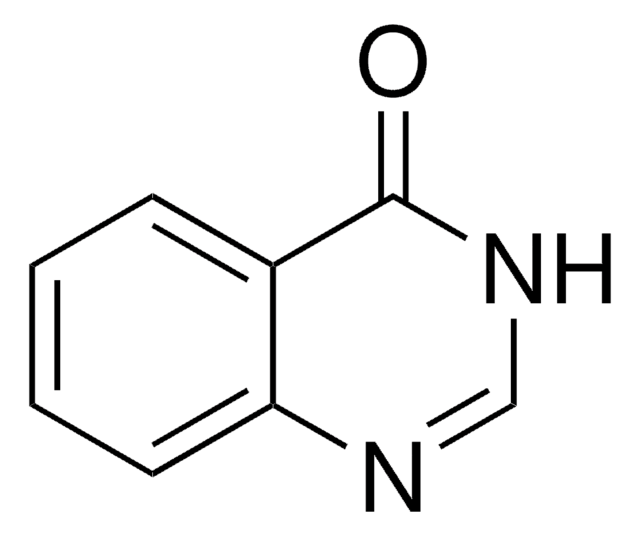
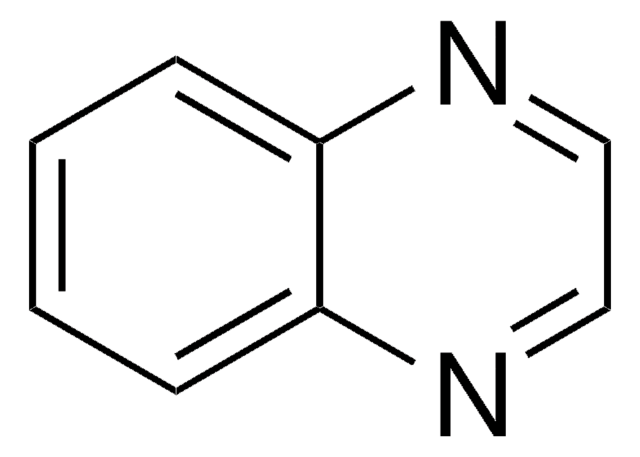
추천 제품
Quality Level
분석
99%
양식
solid
bp
243 °C (lit.)
mp
46-48 °C (lit.)
solubility
H2O: freely soluble
organic solvents: soluble
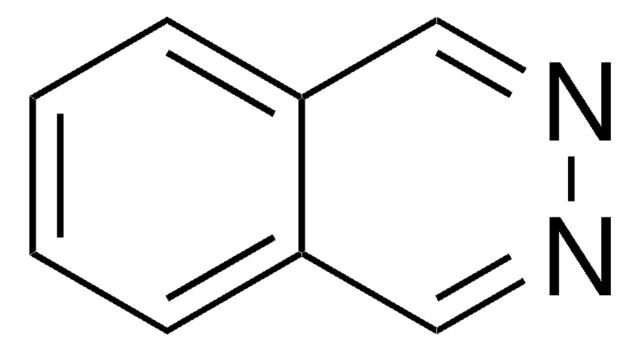
SMILES string
c1ccc2ncncc2c1
InChI
1S/C8H6N2/c1-2-4-8-7(3-1)5-9-6-10-8/h1-6H
InChI key
JWVCLYRUEFBMGU-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Quinazolines has applications in medicinal chemistry due to their antibacterial, antifungal, anticonvulsant, anti-inflammatory and antitumor activities. It is the basic structural unit of pharmaceuticals and plays an important role in modern synthesis of antitumor drugs.
애플리케이션
Quinazoline was used to study the electrochemical behaviour of quinazoline using modern polarographic and voltammetric methods.
생화학적/생리학적 작용
Genotoxicity of quinazoline was established by bacterial SOS Chromotest (Escherichia Coli).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
222.8 °F - closed cup
Flash Point (°C)
106 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
Bruna Possato et al.
Dalton transactions (Cambridge, England : 2003), 46(24), 7926-7938 (2017-06-13)
We report on the investigation of a new series of symmetric trinuclear ruthenium complexes combined with azanaphthalene ligands: [Ru
Kunal Nepali et al.
European journal of medicinal chemistry, 196, 112291-112291 (2020-04-24)
This study reports the design, synthesis and evaluation of a series of histone deacetylase (HDAC) inhibitors containing purine/purine isoster as a capping group and an N-(2-aminophenyl)-benzamide unit. In vitro cytotoxicity studies reveal that benzamide 14 suppressed the growth of triple-negative breast
Reddy Amala et al.
BioImpacts : BI, 11(1), 15-22 (2021-01-21)
Introduction: Inflammation is the primary response caused due to harmful stimuli which are followed by the increased draining of plasma and immune cells from the body into the site of the injured tissue. A signaling cascade of growth factors and
Hamdoon A Mohammed
Medicinal chemistry (Shariqah (United Arab Emirates)), 16(8), 1044-1057 (2020-02-25)
Suaeda is a halophytic genus belonging to the Amaranthaceae family and can survive in the high salted marsh areas of the world. Suaeda plants can biosynthesize natural substances with powerful antioxidant activity and are considered as a renewable source of
Polarographic and voltammetric determination of quinazoline-the structural unit of anticancer drugs.
Hladikova J, et al.
Sensing in Electroanalysis, 3, 165-175 (2008)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.