모든 사진(1)
About This Item
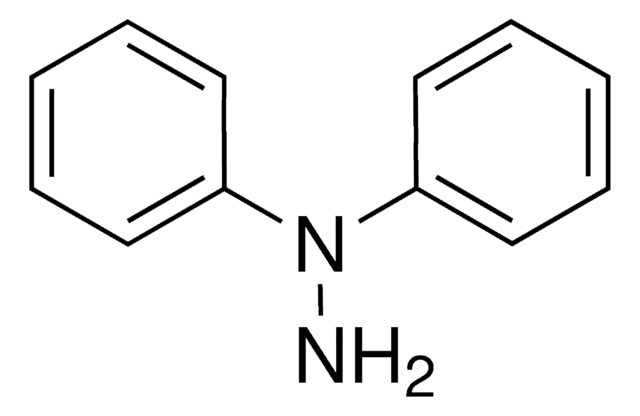
Linear Formula:
C6H5NHNHC6H5
CAS Number:
Molecular Weight:
184.24
Beilstein:
639793
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
형태
solid
Quality Level
mp
123-126 °C (lit.)
SMILES string
N(Nc1ccccc1)c2ccccc2
InChI
1S/C12H12N2/c1-3-7-11(8-4-1)13-14-12-9-5-2-6-10-12/h1-10,13-14H
InChI key
YBQZXXMEJHZYMB-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
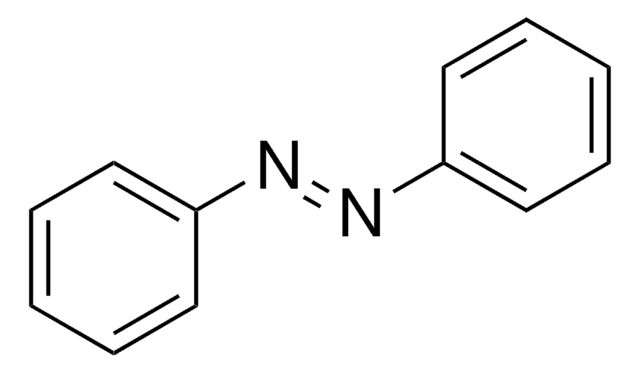
Hydrazobenzene has been prepared by the reduction of azobenzene by SmI(2) in THF.
Hydrazobenzene is an intermediate in the hydrogenation of azoxybenzene to aniline.
애플리케이션
Reactant involved in:
- Insertion reactions with organometallic tantalum complexes
- Reduction reactions catalyzed by titanium(III) trichloride yielding amines
- Studying the mechanism of hydrazobenzene rearrangement
- Reaction with N-heterocyclic stable silylene
- Synthesis of dimanganese amide hydrazide cluster complexes
- Iron-mediated hydrazine reductions yielding iron arylimide cubanes
기타 정보
Contains varying amounts of azobenzene
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
Chintada Nageswara Rao et al.
The Journal of organic chemistry, 76(22), 9438-9443 (2011-10-19)
The reduction of azobenzene by SmI(2) in THF to give hydrazobenzene was investigated. The kinetics are first order in the substrate and first order in SmI(2). The kinetic order in MeOH is ca. 0.56, and in TFE it is ca.
F Matsui et al.
Journal of pharmaceutical sciences, 72(10), 1223-1224 (1983-10-01)
A high-performance liquid chromatographic method has been developed for the simultaneous determination of azobenzene and hydrazobenzene in phenylbutazone and sulfinpyrazone raw materials and formulations. The drug raw material or formulation is shaken with 1N NaOH and n-hexane and centrifuged. The
H Taguchi et al.
Analytical biochemistry, 131(1), 194-197 (1983-05-01)
A new fluorometric assay method for quinolinic acid is introduced in this study. Quinolinic acid-hydrazine complex, a stable fluorescent compound, is formed after heating quinolinic acid with hydrazine at 215-220 degrees C for 2 min. Fluorescence excitation and emission maxima
S Ohnishi et al.
Free radical research, 32(6), 469-478 (2000-05-08)
Hydrazobenzene is carcinogenic to rats and mice and azobenzene is carcinogenic to rats. Hydrazobenzene is a metabolic intermediate of azobenzene. To clarify the mechanism of carcinogenesis by azobenzene and hydrazobenzene, we investigated DNA damage induced by hydrazobenzene, using 32P-5'-end-labeled DNA
Hydrazobenzene.
Report on carcinogens : carcinogen profiles, 11, III146-III147 (2004-01-01)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.