135992
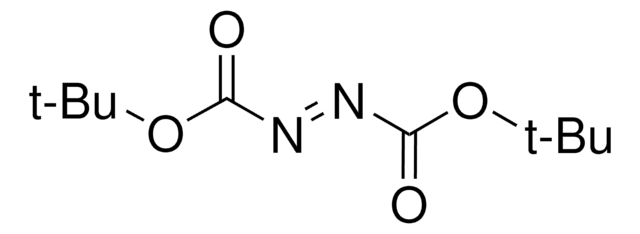
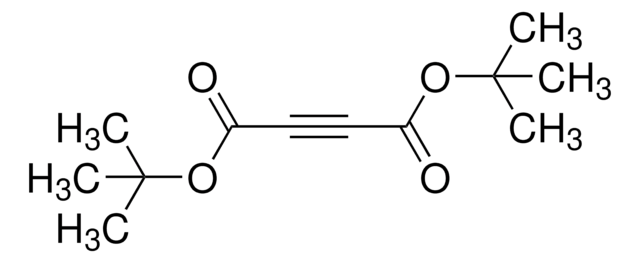
Di-tert-butyl azodicarboxylate
98%
동의어(들):
Bis(1,1-dimethylethyl)azodicarboxylate, DBAD, Di-tert-butyl azodiformate, NSC 109889
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
Linear Formula:
(CH3)3COCON=NCOOC(CH3)3
CAS Number:
Molecular Weight:
230.26
Beilstein:
1911434
EC Number:
MDL number:
UNSPSC 코드:
12352108
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
98%
형태
solid
환경친화적 대안 제품 특성
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
89-92 °C (lit.)
작용기
azo
환경친화적 대안 카테고리
저장 온도
2-8°C
SMILES string
CC(C)(C)OC(=O)\N=N\C(=O)OC(C)(C)C
InChI
1S/C10H18N2O4/c1-9(2,3)15-7(13)11-12-8(14)16-10(4,5)6/h1-6H3/b12-11+
InChI key
QKSQWQOAUQFORH-VAWYXSNFSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for catalysis.
Automate your Mitsunobu reactions with Synple Automated Synthesis Platform (SYNPLE-SC002)
Automate your Mitsunobu reactions with Synple Automated Synthesis Platform (SYNPLE-SC002)
애플리케이션
Co-oxidant in copper-catalyzed greener oxidation of alcohols under aerobic conditions.
Modified Markó′s aerobic oxidation of alcohols under atmospheric pressure with air or molecular oxygen at room temperature
Modified Markó′s aerobic oxidation of alcohols under atmospheric pressure with air or molecular oxygen at room temperature
Reactant for:
- Preparation of hexapeptide key fragments via stereoselective selenocyclization/oxidative deselenylation or hydrazination/cyclization reactions
- Asymmetric Michael addition reactions
- Preparation of dipeptidyl peptidase IV dependent water-soluble prodrugs via Mitsunobu reaction
- Synthesis of pyrroloisoquinoline template via stereoselective N-acyliminium-mediated cyclization and enolate amination for synthesis of peptidomimetic compounds
- Barbier-type propargylation reactions
- Synthesis of bacterial peptide deformylase (PDF) inhibitor fumimycin
- Asymmetric amination of glycine Schiff bases
Reagent employed in the electrophilic amination of ß-keto esters catalyzed by an axially chiral guanidine. Building block in an enantioselective synthesis of 3,6-dihyropyridazines employing organocatalysts such a L-proline or (S)-2-pyrrolidinyl tetrazole.
Utilized in the asymmetric Friedel-Crafts amination via a chiral organocatalyst.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Chirally aminated 2-naphthols--organocatalytic synthesis of non-biaryl atropisomers by asymmetric Friedel-Crafts amination.
Sebastian Brandes et al.
Angewandte Chemie (International ed. in English), 45(7), 1147-1151 (2006-01-04)
Masahiro Terada et al.
Journal of the American Chemical Society, 128(50), 16044-16045 (2006-12-15)
A newly designed axially chiral guanidine is found to function as an effective platform for asymmetric induction at the alpha-carbon of unsymmetrically substituted 1,3-dicarbonyl compounds. Highly efficient and enantioselective electrophilic amination of various 1,3-dicarbonyl compounds with azodicarboxylate was successfully achieved
Synlett, 2548-2548 (2006)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.