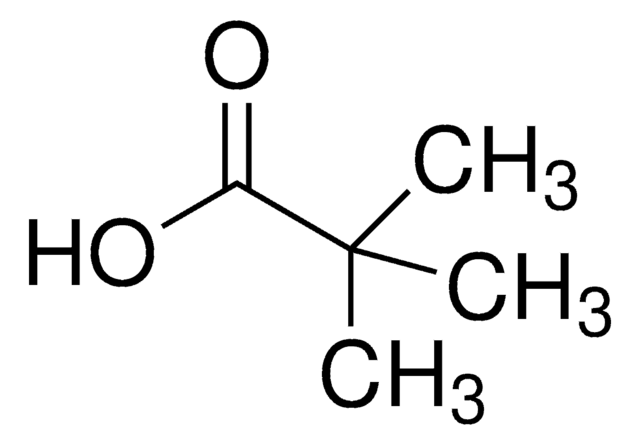
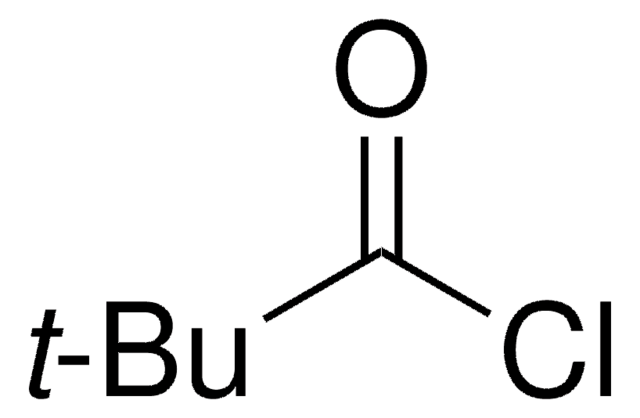
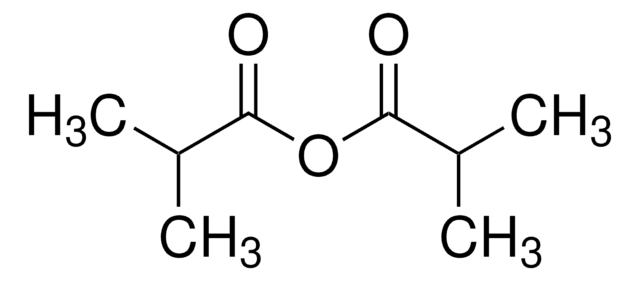
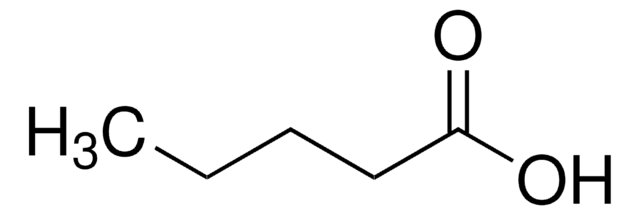
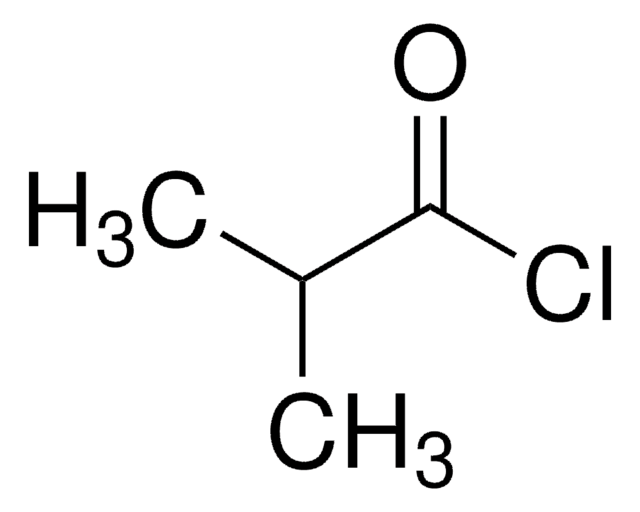
추천 제품
Quality Level
분석
99%
양식
liquid
refractive index
n20/D 1.409 (lit.)
bp
193 °C (lit.)
density
0.918 g/mL at 25 °C (lit.)
작용기
anhydride
ester
SMILES string
CC(C)(C)C(=O)OC(=O)C(C)(C)C
InChI
1S/C10H18O3/c1-9(2,3)7(11)13-8(12)10(4,5)6/h1-6H3
InChI key
PGZVFRAEAAXREB-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Acylation and esterification reagent for anilines and phenols, respectively.
Trimethylacetic anhydride was used:
- in solid-phase oligonucleotide synthesis
- in kinetic resolution of racemic 2-hydroxy-γ-butyrolactones with diphenylacetic acid
- as acylation and esterification reagent for anilines
- as acylation and esterification reagent for phenols
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
162.5 °F - closed cup
Flash Point (°C)
72.5 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
Bulletin of the Chemical Society of Japan, 67, 210-210 (1994)
Alexey Evdokimov et al.
Nucleic acids research, 41(12), e123-e123 (2013-04-24)
DNA probes for the studies of damaged strand excision during the nucleotide excision repair (NER) have been designed using the novel non-nucleosidic phosphoramidite reagents that contain N-[6-(9-antracenylcarbamoyl)hexanoyl]-3-amino-1,2-propandiol (nAnt) and N-[6-(5(6)-fluoresceinylcarbamoyl)hexanoyl]-3-amino-1,2-propandiol (nFlu) moieties. New lesion-imitating adducts being inserted into DNA show
Australian Journal of Chemistry, 60, 75-75 (2007)
Q Zhu et al.
Bioorganic & medicinal chemistry letters, 11(9), 1105-1107 (2001-05-17)
Commercially available 'fast-deprotecting' phosphoramidites are useful for synthesizing oligonucleotides containing alkali-sensitive nucleotides. However, N-acetylated oligonucleotides were observed during solid-phase synthesis using 'fast-deprotecting' phosphoramidites in conjunction with K2CO3/MeOH ('ultra-mild') deprotection. Transamidation was localized at deoxyguanosine, which is protected as its isopropylphenoxyacetyl
Z J Kamiński
International journal of peptide and protein research, 43(3), 312-319 (1994-03-01)
According to the concept presented, esters forming an amide (peptide) bond by the mechanism SN#DN or SN#*DN involving fast decay of the tetrahedral intermediate may behave as 'superactive acylating reagents'. These should render coupling involving less reactive substrates, i.e. sterically
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.