추천 제품
vapor pressure
0.01 mmHg ( 25 °C)
Quality Level
분석
99%
형태
solid
bp
260-262 °C (lit.)
mp
39-42 °C (lit.)
solubility
alcohol: freely soluble
benzene: freely soluble
chloroform: freely soluble
diethyl ether: freely soluble
petroleum ether: very slightly soluble
water: very slightly soluble
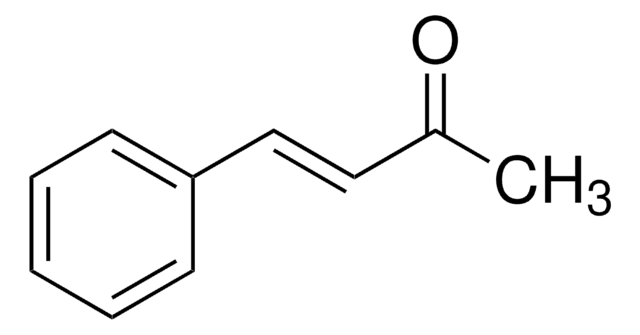
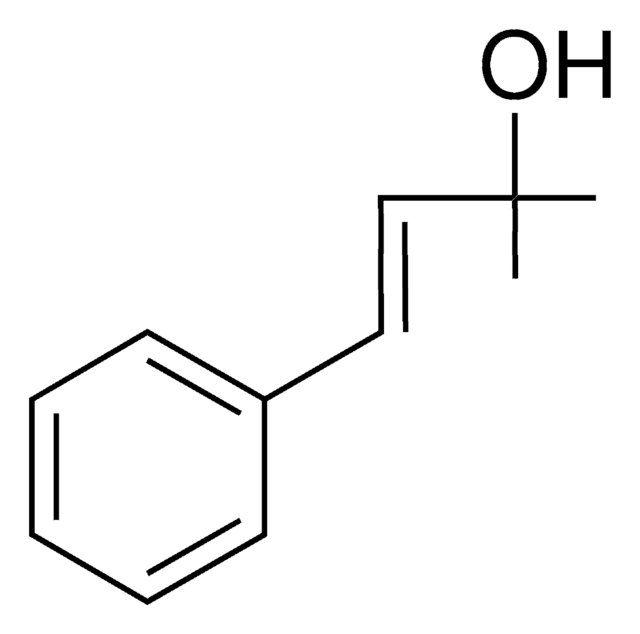
SMILES string
[H]\C(=C(\[H])c1ccccc1)C(C)=O
InChI
1S/C10H10O/c1-9(11)7-8-10-5-3-2-4-6-10/h2-8H,1H3/b8-7+
InChI key
BWHOZHOGCMHOBV-BQYQJAHWSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
trans- 4-Phenyl-3-buten-2-one is substrate for glutathione transferase. It reacts with methyl- and benzylguanidine to yield aromatic N2-substituted 2-pyrimidinamines.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
253.4 °F - closed cup
Flash Point (°C)
123 °C - closed cup
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
이미 열람한 고객
Dihydropyrimidines and related structures. I. N2-substituted 2-pyrimidinamines and dihydro-2-pyrimidinamines by reaction of phenylbutenones and monosubstituted guanidines.
Winfried W and Schermanz K.
Journal of Heterocyclic Chemistry, 21(1), 65-69 (1984)
Glutathione S-transferases. The first enzymatic step in mercapturic acid formation.
W H Habig et al.
The Journal of biological chemistry, 249(22), 7130-7139 (1974-11-25)
Bowon Kwon et al.
Journal of economic entomology, 101(1), 36-41 (2008-03-12)
Benzylideneacetone (BZA) is a metabolite of gram-negative entomopathogenic bacterium Xenorhabdus nematophila, and it acts as an enzyme inhibitor against phospholipase A2 (PLA2). PLA2 catalyzes a committed biosynthetic step of eicosanoids, which mediate insect immune reactions to infection by microbial pathogens.
Chisako Yamagami et al.
Bioorganic & medicinal chemistry letters, 14(22), 5629-5633 (2004-10-16)
Antioxidant activities for a series of hydroxybenzalacetones, OH-BZ, were evaluated by measuring inhibitory potencies of OH-BZ against lipid peroxidation induced by t-BuOOH or gamma-irradiation. Their quantitative structure-activity relationship (QSAR) studies indicated that the activities are mainly governed by electronic and
Hiroyuki Morita et al.
Proceedings of the National Academy of Sciences of the United States of America, 107(2), 669-673 (2010-01-19)
Benzalacetone synthase (BAS), a plant-specific type III polyketide synthase (PKS), catalyzes a one-step decarboxylative condensation of malonyl-CoA and 4-coumaroyl-CoA to produce the diketide benzalacetone. We solved the crystal structures of both the wild-type and chalcone-producing I207L/L208F mutant of Rheum palmatum
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.