W288101
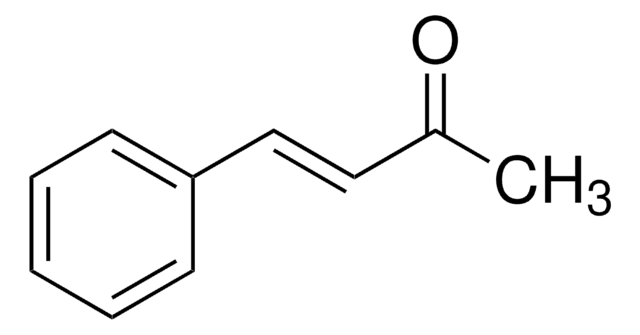
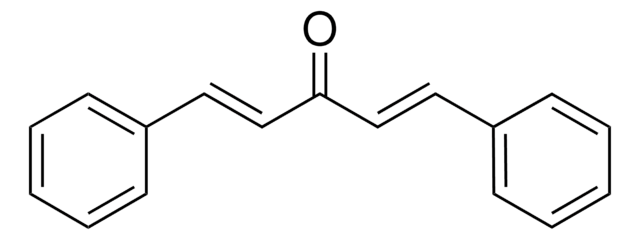
Benzylideneacetone
≥98%, FG
동의어(들):
Benzalacetone, 4-Phenylbut-3-en-2-one, Benzylideneacetone, Methyl styryl ketone
About This Item
Halal
Kosher
추천 제품
생물학적 소스
synthetic
Quality Level
Grade
FG
Halal
Kosher
Agency
meets purity specifications of JECFA
규정 준수
EU Regulation 1334/2008 & 872/2012
FDA 21 CFR 172.515
vapor pressure
0.01 mmHg ( 25 °C)
분석
≥98%
bp
260-262 °C (lit.)
mp
39-42 °C (lit.)
응용 분야
flavors and fragrances
문건
see Safety & Documentation for available documents
식품 알레르기항원
no known allergens
감각 수용성의
anise; cinnamon; jam; balsamic; spicy; floral; sweet
SMILES string
[H]\C(=C(\[H])c1ccccc1)C(C)=O
InChI
1S/C10H10O/c1-9(11)7-8-10-5-3-2-4-6-10/h2-8H,1H3/b8-7+
InChI key
BWHOZHOGCMHOBV-BQYQJAHWSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
생화학적/생리학적 작용
기타 정보
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
253.4 °F - closed cup
Flash Point (°C)
123 °C - closed cup
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.